Role of Periodic Input Composition and Sweeping Gas for Improvement of Hydrogen Production in a Palladium Membrane Reactor by Partial Oxidation of Methane
Lemnouer Chibane* and Brahim Djellouli
Laboratoire de Génie des Procédés Chimiques (LGPC), Université Ferhat Abbas, Sétif, 19000 Algérie
1 INTRODUCTION
Hydrogen is one of the most important compounds in the chemical and petrochemical industries.It is used in various applications such as the production of certain chemical products like methanol, OXO synthesis or in the Fischer-Tropsch process [1, 2] and ammonia synthesis. Because hydrogen is an energy vector which can be stored and does not generate any pollutants such as carbon dioxide, vast hopes are placed on this vector. Actually, hydrogen energy could find significant applications in the transportation sector and distributed power generation, and would further facilitate renewable energy implementation by acting as a storage medium [3]. Because of its high efficiency, the fuel cell is often considered as one of the main drivers for hydrogen as a fuel of the future[4]. The vast majority of automotive fuel cell applications rely on polymer electrolyte membrane (PEM)fuel cells, which have proven to be highly efficient in converting hydrogen to power. Several routes are possible to produce hydrogen including water electrolysis[5], by natural gas reforming [6-8] or by steam reforming of liquid hydrocarbons [9-11]. Moreover, hydrogen can be produced by other means, such as from biomass [12] or by the solar thermal dissociation of water at high temperature [13]. Thus, thermochemical reforming techniques are widely used to produce hydrogen from hydrocarbon fuels.
The partial oxidation of methane reaction appears as an alternative process for hydrogen production.This reaction, which has been investigated by several researchers [1, 14-17] presents a low exothermic balance and produces favorable ratio of hydrogen to carbon monoxide. According to Dong et al. [1] and Lu et al. [2], the lower H2/CO ratio presents a perfect stoichiometry for the Fischer-Tropsch process. De Groote and Froment [16] reported that the advantage of this reaction is the possibility of using it with a desired molar ratio of oxygen to methane in the feed(O/C). Feeding only oxygen and methane leads to syngas with low H2/CO ratio, but coke deposition is difficult to avoid [16]. Basile et al. [15] reported that the molar ratio H2/CO in the exit of the reactor could be adjusted by the O/C molar ratio. The reaction temperature plays an important role in the reactor performance via thermodynamics and kinetics. The global thermodynamics of methane partial oxidation was studied by Christian et al [18]. They studied the effect of temperature and pressure on a stoichiometric mixture of methane and oxygen (O/C=0.5). They found that high temperatures are required with increasing pressures to obtain high conversion, and high selectivity to hydrogen and carbon monoxide. But operating at high pressure and much higher temperatures in the case of Pd-membrane reactor are not favorable. However, the Pd-based membrane has some limitations including mainly embrittlement and thin films that are free of cracks or pinholes. According to Galuszka et al.[19] and Chen et al. [20], palladium membranes are not suitable for high temperature reactions because of the formation of carbon filaments and pinholes during the reaction, which destroys the metallic layer.
One possible way to enhance the performance of this reaction and thus attain a high methane conversion at lower temperatures is to use membrane reactors [21].Membrane reactors could be used in the process to overcome the equilibrium limitation by selectively removing hydrogen from the reaction zone [22]. The membrane continuously removes the hydrogen produced in the catalytic reaction zone thus pushing the chemical equilibrium and allowing higher methane conversion at a lower temperature [23]. The use of membrane reactors appears to be an interesting way to improve hydrogen yield at lower temperatures because the removal of hydrogen from the reaction environment prevents the equilibrium to be achieved [24]. The membranes used in catalytic reactors are generally characterized with a high permeability and a good selectivity of separation, and they are stable at the reaction temperature especially in the presence of gas.Among hydrogen selective membranes, Pd-membranes remain the most promising Pd-based membrane reactors and are considered in most of the studies because they would produce pure hydrogen and thus simplify the conventional operation including extensive hydrogen purification steps [25]. One way for the enhancement of membrane reactor performance is to use a sweep gas to accelerate the transport of hydrogen through the membrane. But the employment of a large quantity of sweeping gas is economically undesirable in the industrial process [4, 26], and causes problems of hydrogen separation from the sweeping gas (dilution problems) [24].
In order to improve the performance of the reaction, another new way can be applied using the ultrasound perturbation. There are many advantages of this technique. It gives good yields, influences the selectivity of reaction, and gives short reaction times [27].In general, the ultrasonic waves have a low-amplitude and a high frequency [28]. Our work is oriented toward forcing a manipulated variable or more at low frequencies by periodic operations that are primarily an engineering tool for the control of conversion or selectivity in a chemical reactor [29]. The attractiveness of the periodic operations is related to the fact that the average process performance corresponding to the periodic operation can be superior to that at the optimal steady state [30]. There are many ways of operating a reactor periodically. A reactor input is a manipulated variable and almost all inputs can be forced periodically. For example, the conversion of SO2in the presence of air and water can be increased by about 50% if the water flow is periodically turned on for a short time rather than flowing continuously through the bed [29]. The total pressure modulation can also be used to improve the reactor performance and increase the rate of transport. It has been proven that fast periodic temperature variation may increase the reaction rate compared to the stationary temperature conditions[31]. Moreover, other advantages of catalytic reactor operation by composition forcing include increased conversion, improved selectivity, and reduced catalyst deactivation [29]. The periodic operation of chemical membrane reactors used for hydrogen production is a new concept and the amplitude effects have not been heavily explored in periodically operated membrane reactors. Our attention is focused on enhancing the performance of a Pd-membrane reactor used for pure hydrogen production from partial oxidation of methane. The present study is limited to the case when the reaction input composition and sweeping gas are running periodically.
2 MATHEMATICAL MODEL
2.1 Chemical reaction scheme and kinetics
In this study, the partial oxidation of methane is carried out in a Pd-based membrane reactor where many reactions are taking place and whose rates depend strongly on the reforming conditions. The process reaction scheme is as follows [15] and includes the exothermic complete combustion of a fraction of feed of methane followed by the endothermic methane steam reforming and other reactions:
Total combustion of methane:
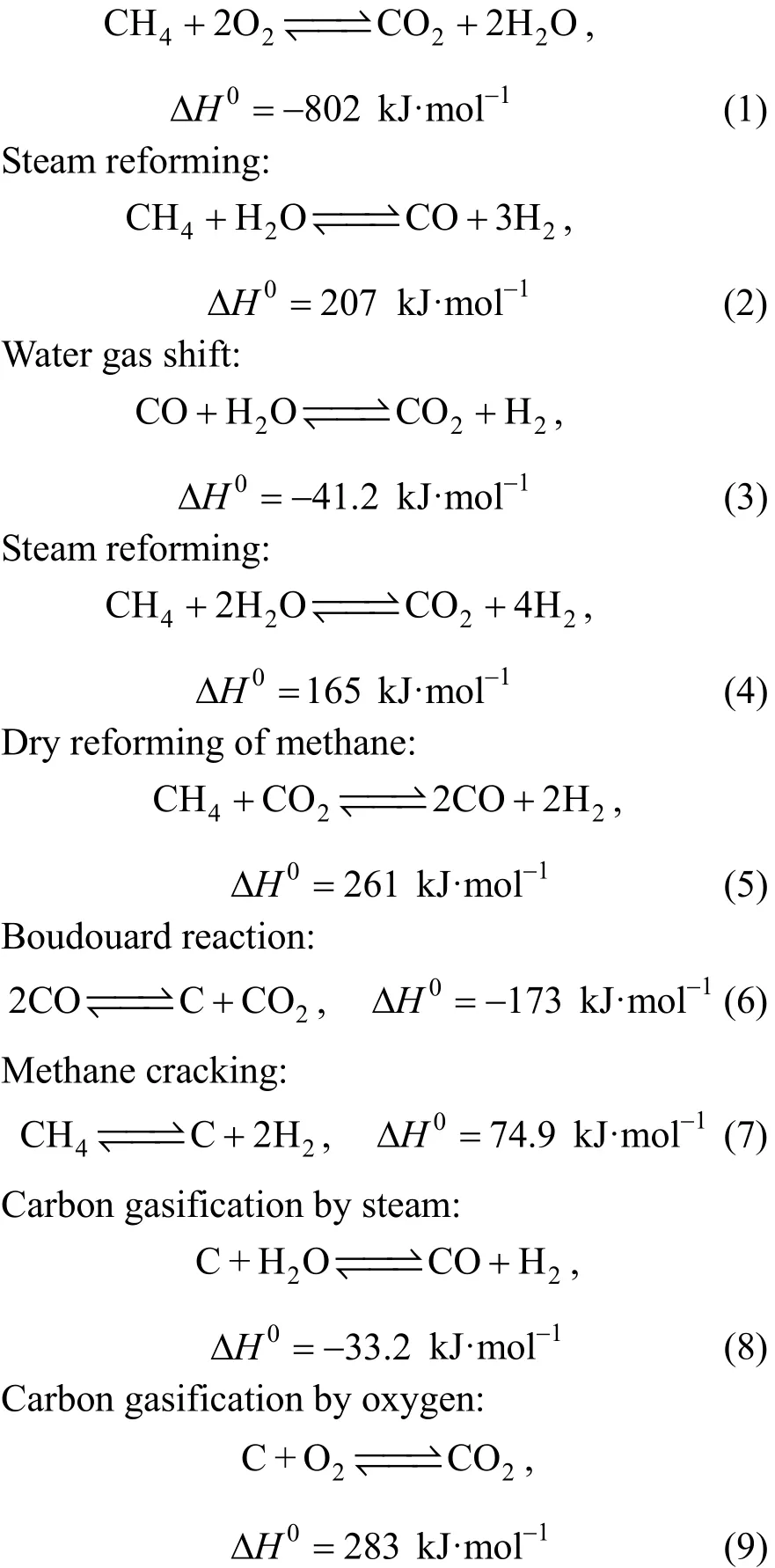
To reduce the complexity of the mathematical model development and solution, only the reactions with significant rates will be considered. Among the possible set of reactions previously cited, the total combustion reaction (1), the steam reforming reactions (2) and (4), and the water-gas-shift reaction (3)have been proven to have significant rates [32]. The other reactions [reaction (5) through reaction (9)] are negligible. In this study, we consider only the first four reactions because the coke deposition is supposed to be negligible because of the higher catalyst stability and lower temperature [15]. Hence, the catalytic activity is an important factor in the membrane reactions[33]. In the current study, the Langmuir-Hinshelwood model [34] was chosen for the reaction of the complete oxidation of methane [reaction (1)]. The rate expressions for steam reforming and water gas shift reactions[reactions (2), (3) and (4)] were based on the analysis of Xu and Froment [35]. In this work, Ni/Alumina was assumed for the partial oxidation of methane. Some kinetic equations for the methane total oxidation [reaction (1)] were reported by Trimm and Lam [36], and Maet al[34]. The kinetic of Trimm and Lam was also applied to describe the combustion of methane in a packed bed reactor. This expression was determined with experiments on a Pt/Al2O3catalyst and has been corrected for a Ni catalyst by De Smetet al[37]. The nickel-based catalysts are very effective for the partial oxidation of methane to syngas because of the high turnover rates and the low cost [38].
Kinetic rate equations for the steam reforming and water gas shift reactions (r2,r3andr4) are based on the analysis of Xu and Froment [35]. It must be noted that the kinetic rate expression of Trimm and Lam [36] has been developed at a temperature of 557 °C, but the model of Xu and Froment over Ni-based catalysts is considered more general and has been extensively tested under lab-scale conditions with a temperature range from 500 to 575°C [39].
The reaction ratesrj(j=1, 2, 3 and 4) of reaction(1) to (4) are:
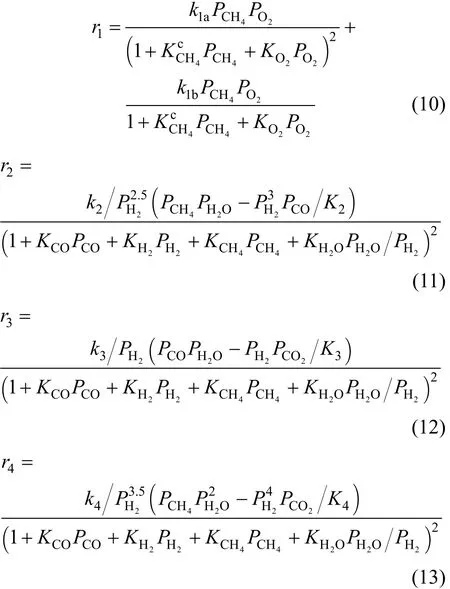
wherekj(j=1, 2, 3 and 4) is the reaction rate constant of reactionjdefined by Arrhenius equation:Kj(j=2, 3 and 4) is the equilibrium constant defined by Van’t Hoff equation:
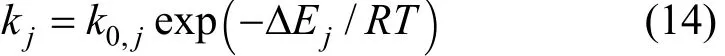
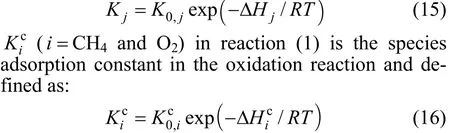
The adsorption constants of species (i=CO, H2, CH4and H2O) in reactions (2), (3) and (4) are defined by the following equation:
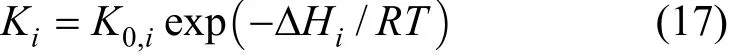
whereRis the universal gas constant andTis the temperature.4CHP,2OP,2HP,COP,2HOP, and2COPare the partial pressures of CH4, O2, H2, CO, H2O and CO2,respectively. The kinetic and thermodynamic data used for the simulation are summarized in Tables 1 to 3.
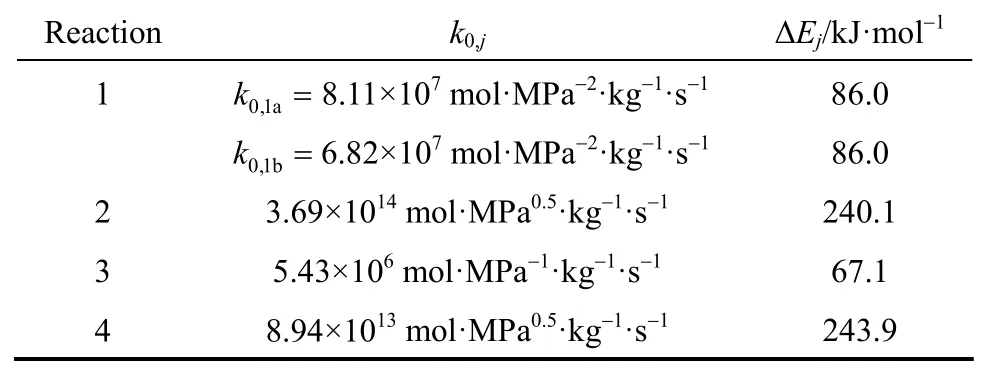
Table 1 Arrhenius kinetic parameters
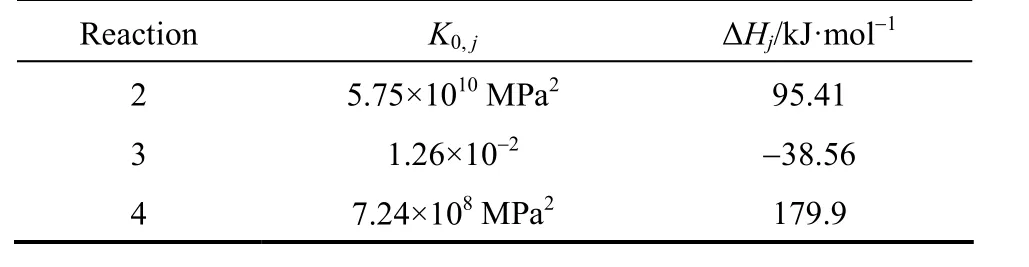
Table 2 Van’t Hoff parameter values for equilibrium constants

Table 3 Van’t Hoff parameter values for species adsorption
2.2 Membrane and reactor description
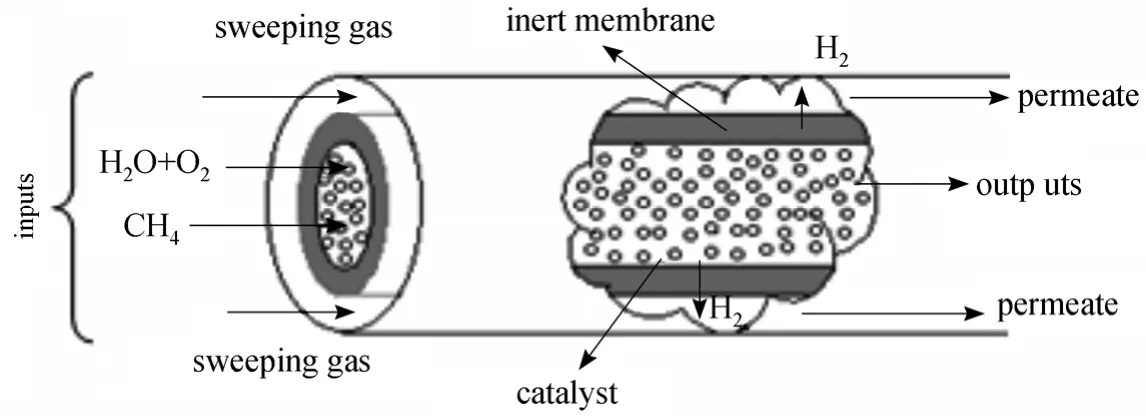
Figure 1 Schematic representation of the membrane reactor
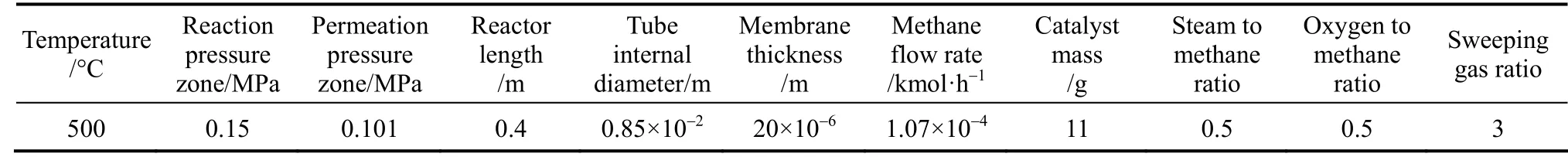
Table 4 Reactor dimensions and operating conditions
The membrane used [6] is a dense palladium membrane with a thickness ofδ=20 μm and it is only permeable to hydrogen. The reactor studied (Fig. 1)has an internal diameterD=0.85 cm, and a lengthL=0.4 m. It is noteworthy that according to Hoang and Chan [40], a reactor with a small diameter and a great length (L/D>25) gives better performance in terms of high methane conversion. In this work, the computation data concerning the dimensions of the reactor and the catalyst were taken from Halabiet al.[41], and the reactor used consists of two concentric tubes. The external tube is a stainless steel tube and the internal one is an inert membrane. The mass of Ni/γ-Al2O3catalyst used is 11 g. It is regularly arranged inside the internal tube. The reactants are axially injected inside the inner tube through the catalyst. After the catalytic reaction, the hydrogen produced diffuses through the membrane into the outside tube where it is evacuated by a sweeping gas (or with an inert gas).
For the simulation, a model is developed to investigate the partial oxidation process behavior at steady state and under periodic operation conditions in a fixed bed membrane reactor to produce pure hydrogen. The simulated operating conditions are presented in Table 4.
The mathematical model used is based on the following assumptions:
(1) The membrane reactor is operated under isothermal and isobaric conditions.
(2) The catalyst deactivation by coke formation is negligible.
(3) The periodic operation is only imposed for the steam to methane ratio, oxygen to methane ratio and sweeping gas ratioviaa square and a sinus ways.
(4) Under forcing conditions, it is supposed that the period of change of the modulation function is significantly larger than the characteristic process time, so the reactor operates under quasi steady state conditions.
(5) Palladium and palladium composite membranes are used only when ultra-pure hydrogen is needed. Therefore, we assume that the membrane is only permeable to hydrogen.
2.3 Hydrogen transport in the Pd membrane
The hydrogen permeation through the Palladium membrane with a thicknessδfollows a particular mechanism of molecular hydrogen dissociation and atomic diffusion. The hydrogen permeated depends on not only the membrane properties but also a linear function of the driving force [42]. The rate of hydrogen permeation can therefore be expressed as a function of the difference in the square root of hydrogen partial pressures on both sides of the membrane. A permeation law resulting from a mechanism of solutiondiffusion allows us to express the term of permeation by the following equation [43]:
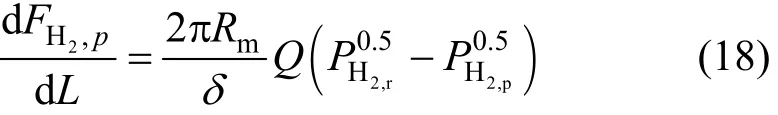
where2,rHPand2,pHPare the hydrogen partial pressures in the reaction side and permeation side, respectively.Rmis the membrane radius and2,pHFis the flow rate of the hydrogen in permeate zone.
On the other hand, the hydrogen recovery (2HY)is defined as the flow rate of the hydrogen in permeate zone (2,pHF) to the initial flow rate of methane in reac-

The hydrogen permeation coefficient is a strong function of temperature and can be described by Arrhenius equation as follows:
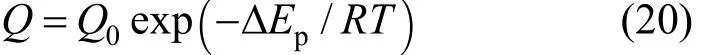
By substituting2,pHFandQgiven by Eqs. (19) and(20) in Eq. (18) and by introducing the dimensionless form d d/ZLL= , the following expression can be obtained:
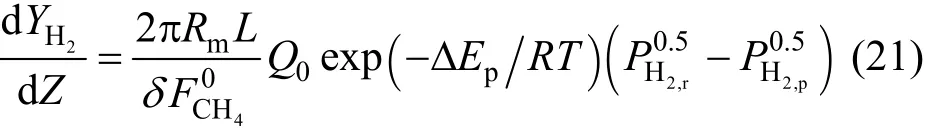
where the apparent activation energy ispEΔ=29.73 kJ·mol-1and the pre-exponential factor is Q0=7.7×10-5mol·m·(s·m2·kPa0.5)-1[15].
The hydrogen pressure in the permeation zone PH2,pis defined by:
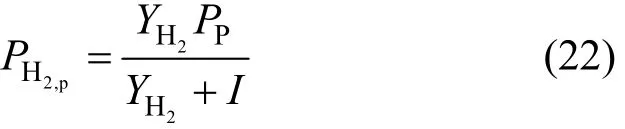
I is the sweeping gas ratio defined as the ratio of the sweeping gas flow rate ()to that of methane at the inlet of the catalyst bed ().

It is obvious that the hydrogen partial pressure in the permeation zone depends on the sweeping gas ratio.Any increase in the sweep ratio leads to a decrease of the hydrogen partial pressure in the permeation side.
2.4 Mass balances
The rate of consumption or formation of each species is given by the following expressions. In addition, in order to take into account the intra-particle mass transport limitations, average reaction rates are computed. The average rates are determined by multiplying the rates [Eqs. (10) to (13)] by various effectiveness factors;1η=0.05,2η=0.07,3η=0.7 and 4η=0.06, respectively [16]:
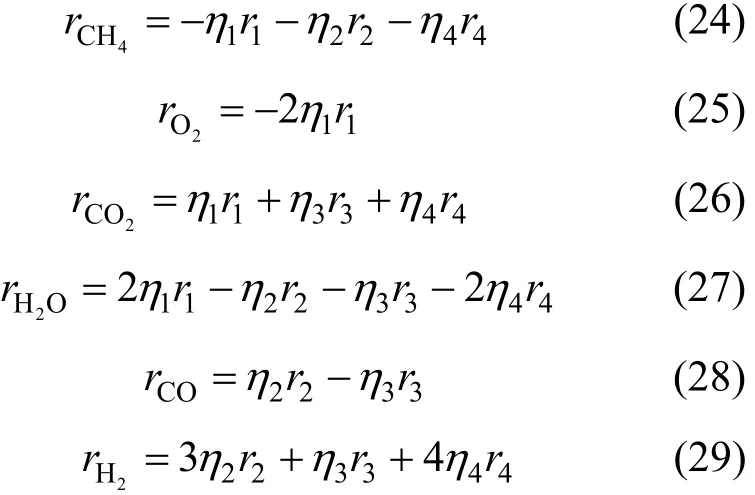
The axial differential mass balance in the fluid phase is given for each component by the following expression:
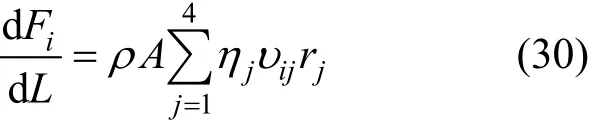
where A is the reactor section (m2), ρ is the catalyst density (kg·m-3),ijυ is the stoichiometric coefficient of component i of reaction j, andjη (j=1, 2, 3 and 4)is the effectiveness factor for reactions (1), (2), (3) and(4), respectively.
The mass balance relative to the methane can be written as:

According to the reactions stoichiometry, the disappearance rate of methane is equal to the sum of its disappearance rates in the first, the second and the fourth reactions. Eq. (31) can be further expressed by the introduction of a dimensionless reactor length Z,d d/Z LL= . Hence, we obtain:
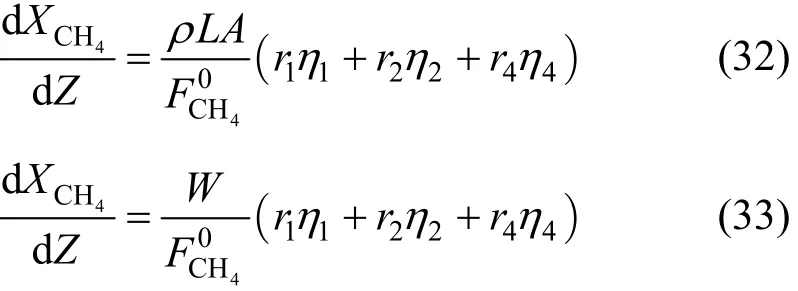
The oxygen disappearance rate can be expressed as follows:
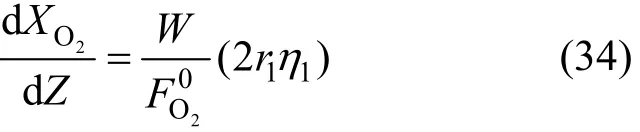
Moreover, the rates of the formations of H2O, CO2and CO are given by:
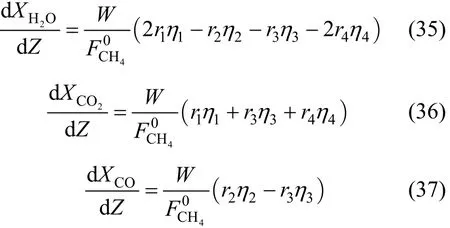
2.5 Concept used for modulation
In this work, a periodic reactor operation given previously is considered for the reaction of the partial oxidation of methane. The reaction is characterized by a mild exothermicity and a very short residence time on the surface of the catalyst [44]. In a periodic operation, an input that affects the system performance is selected for manipulation (modulation) [45]. The concept of using a membrane reactor with a periodic operation to enhance the hydrogen recovery during the partial oxidation of methane is investigated. It is assumed that the composition (steam and oxygen) and sweeping gas ratio imposed feature a steady component and a time dependent component under the periodic operations.
The cycle is described by a period 2τ=π, an amplitude σ and a frequency ω. The modulation functions at a constant inlet feed of methane ( F0=constant)
CH4can be written using a sinus and a square functions as follows:
Periodic operation with a sinus function f1(t):
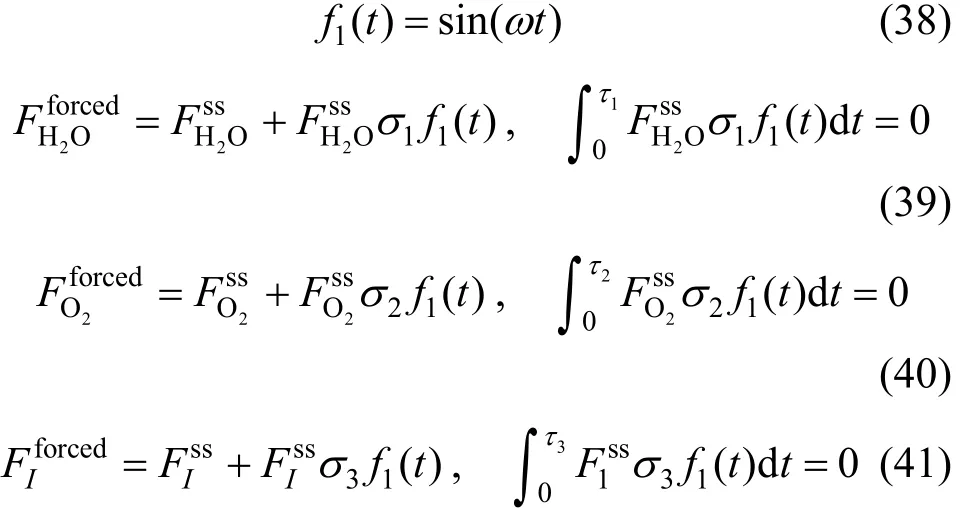
Periodic operation with a square function f2(t):

Thus, at 0t= , the integral values under periodic operations (forced) are equal to those in steady state (SS).
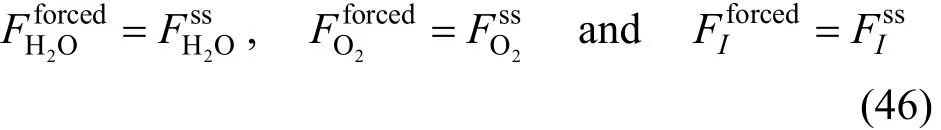
where1σ,2σ and3σ are the running amplitudes of the steam, oxygen and sweeping gas, respectively. The frequency used in the periodic operation is limited by the product properties. Low frequencies may be used if the characteristic time of the reactor is constant. On the other hand, high frequencies may force the reactor into a relaxed steady state condition [45].
τ1, τ2and τ3are the periods of change of the modulation function. It is supposed in this work that these periods are significantly larger compared to the characteristic times of the reactor. Even though the operation is periodic, the average reaction time is uniquely determined by the steady state behavior of the reaction or reactor. The term “quasi steady state” is commonly used for this mode [29]. Since the composition affects the effectiveness factors, it is assumed that the effectiveness factors are constant in order to isolate the effect of cycling.
The reactor performance was evaluated through the following ratios (normalized) defined as the ratio of the methane conversion, hydrogen recovery and H2/CO computed under forcing conditions (forced) to steady state conditions (SS), respectively:
Normalized conversion:
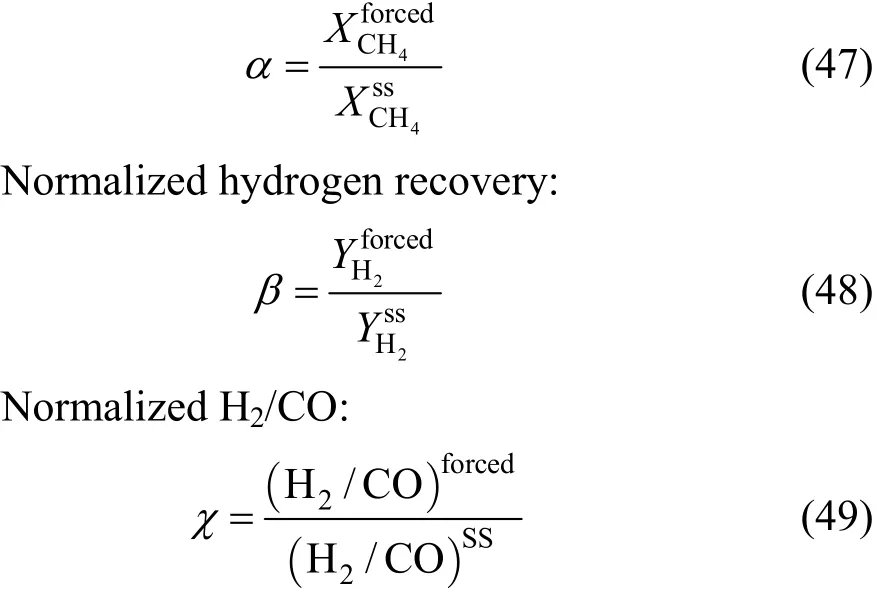
3 SIMULATION RESULTS AND DISCUSSION
3.1 Solution procedure
As mentioned above, the objective of this work is to study the partial oxidation of methane in a Pd-based membrane reactor under a moderate temperature (500 °C)and pressure (0.15 MPa) using the concept of modulation. The procedure followed to study the partial oxidation of methane process at a milder temperature begins by the computation of the hydrogen recovery through the Palladium membrane [Eqs. (18) to (23)] and the calculation of the methane conversion using equations obtained from the mass balances in the fluid phase[Eqs. (33) to (37)]. In the case of periodic operations,the set of equation [Eqs. (38) to (46)] is added. These equations were solved numerically by the Runge-Kutta method. An analysis of the effect of steam to methane ratio (S/C), oxygen to methane ratio (O/C) and sweeping gas ratio (I) under forcing conditions was presented by a mathematical model. Those inputs are modulated periodically around their steady state values (S/C=0.5,O/C=0.5 and I=3).
The kinetics of the partial oxidation reaction presents a critical parameter, the hydrogen partial pressure (steam reforming reaction). This parameter is adjustable and can be computed by trying values from zero to some values. Because of kinetic limitations,zero cannot be used as a value for hydrogen partial pressure because it will give us infinite kinetics. To overcome this problem, a small quantity of hydrogen has to be used. In addition, a mixture of methane and steam (inlet gas) is used for improving hydrogen yield and reducing carbon monoxide [40].
3.2 Case of steady sate conditions
In general, the partial oxidation of methane operates under O/C ratio equal to 0.5 and typically at low S/C ratio [41]. In order to determine the optimum S/C corresponding to the maximum of hydrogen recovery at O/C=0.5, an analysis was made. The O/C molar ratio appears as the key parameter in this reaction because it determines and governs the CO formation. Thus, the effect of this ratio on the variation of methane conversion, hydrogen recovery and the H2/CO ratio was studied. The results obtained showed that at a fixed molar methane flow rate (1.07×10-4kmol·h-1), the methane conversion increases with increasing O/C and S/C molar ratios. The maximum hydrogen recovery depends on the optimal O/C ratio. At several ratios of both O/C and S/C, ranging from 0.1 to 1, it was found that the hydrogen recovery increases with increasing S/C ratio. Therefore, the reaction has an optimal O/C ratio giving a maximum hydrogen recovery. It was found that it is appropriate to switch between O/C and S/C ratio in order to obtain a better performance in term of hydrogen recovery. There is an optimal pair of(O/C, S/C) that leads to a maximum of hydrogen recovery. The results showed that using a lower S/C ratio required the use of a large O/C ratio andvice versa.
The results also showed that any increase of S/C ratio from 0.1 to 1 and O/C ratio from 0.1 to 1 provokes an increase of the conversion of methane. Thus,from the point of view of hydrogen production (hydrogen recovery), a higher O/C ratio allowed higher hydrogen recovery (2HY). However, higher H2/CO ratios were also obtained. In general, this ratio is adjustable by the O/C ratio inlet. It was also found that at lower steam to methane ratio varying from 0.1 to 0.3, the O/C ratio required for obtaining a maximum hydrogen recovery changed from 0.7 to 0.6, respectively. With increasing the S/C ratio from 0.4 to 0.6(medium S/C ratio), the O/C ratios required for obtaining higher hydrogen recovery are varied from 0.5 to 0.4, respectively. At higher S/C ratios ranging from 0.7 to 1, it was found that the O/C ratio of 0.3 to 0.2 is satisfactory to obtain high hydrogen recovery.
From these results, it is concluded that using higher S/C ratios is favorable to obtain a higher hydrogen recovery, but higher H2/CO ratios were also obtained. The control of H2/CO molar ratio is vital to the downstream application for synthesis gas. Forinstance, Fischer-Tropsch synthesis requires lower ratios of H2/CO [1, 2]. The maximum hydrogen recovery obtained at O/C=0.5 required the use of an S/C ratio ranging from 0.4 to 0.5. However, a large amount was obtained with (S/C, O/C)=(0.5, 0.5). Under these conditions, with the H2/CO ratio being equal to 2.62,the achieved conversion of methane was almost equal to 0.77 and the hydrogen recovery was almost equal to 1. The main results are summarized in Table 5.
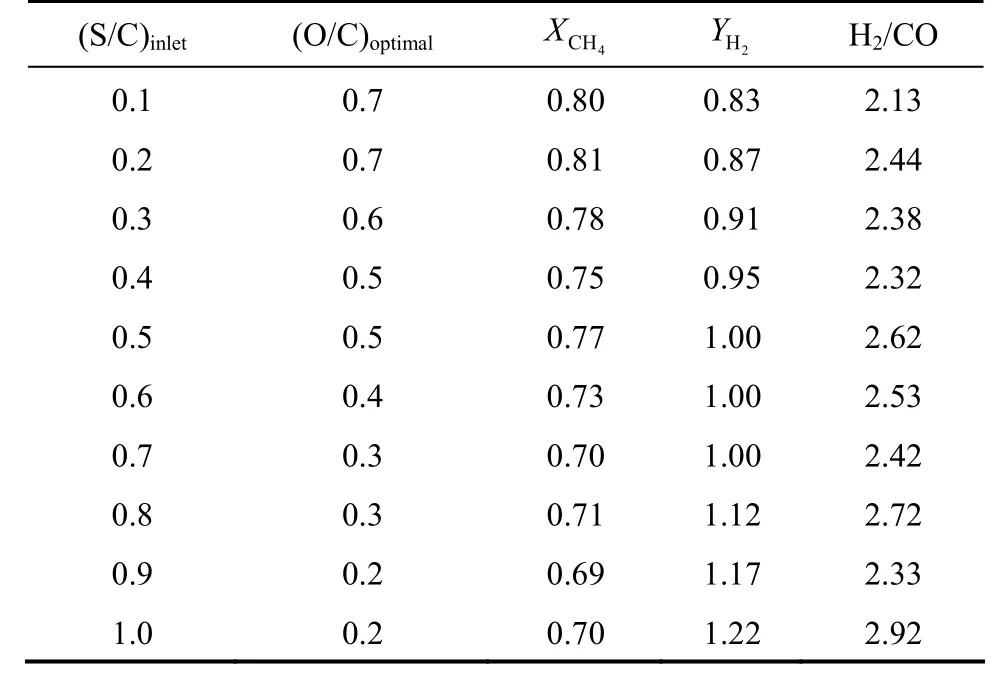
Table 5 Reactor performance under steady state conditions(T=500 °C, Pr=0.15 MPa, Pp=0.1 MPa)
3.3 Analysis under periodic operation conditions
In this work, all the periodic operations are conducted at a fixed methane inlet flow rate. Without of periodic operations for the methane inlet flow rate, the reaction system has three manipulated inputs: steam to methane ratio (S/C), oxygen to methane ratio (O/C)and sweeping gas ratio (I). Here, all the inputs are switched periodically. The inputs flow in the reactor feed is periodically varied in a sinus way and by square wave functions. The strategy followed [29]presents many different possible periodic operations.The first manipulation is to force one input and hold the remaining two inputs constants. In the second manipulation, two of the inputs are forced while one is held constant. The third operation consists of manupilating all three inputs with two cycles exhibiting changes both in phase while the third one is 180° out of phase. In the present study, all the inputs are modulated periodically around their steady state values and only the most significant results are presented. The study begins by the analysis of the effect of modulation of the manipulated inputs used.
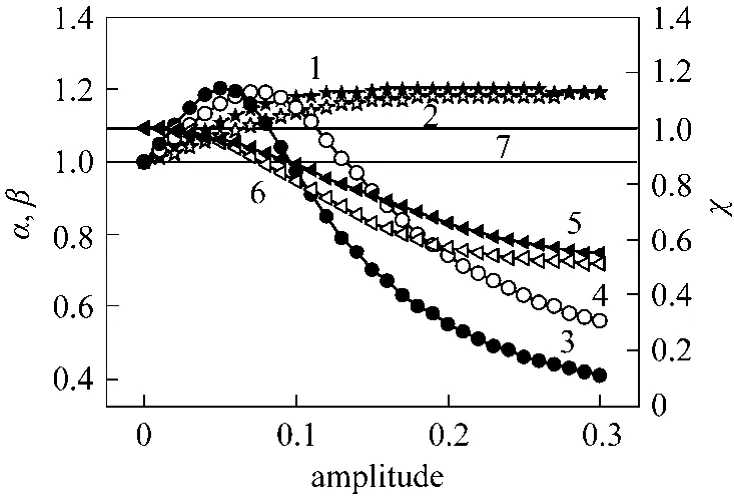
Figure 2 Effect of S/C ratio modulation on the reactor performance1, 2—normalized conversion (α) for square and sinus ways;3, 4—normalized hydrogen recovery (β) for square and sinus ways; 5, 6—normalized H2/CO (χ) for square and sinus ways;7—steady-state conditions
In a first operation, the S/C ratio was forcedviaa square and a sinus functions keeping the sweeping gas ratio (I) and O/C ratio constant. At a fixed molar methane flow rate, the S/C ratio must follow the square and the sinus functions. The results presented in Fig. 2 show the effect of amplitude modulation on the performance of the reactor. It was found that for both modulation modes, the conversion of methane increases slightly when the amplitude increases up to 0.15 and 0.20, respectively. The conversion remains constant with further increase in the amplitude.Therefore, there was an improvement in conversion of methane. The enhancement factors of conversion for the square and sinus modes are 1.20 and 1.18 times higher than those found under steady state conditions,respectively. The hydrogen recovery exhibits a maximum at an amplitude of 0.05 when the periodic operation was runningviaa square mode, but at an amplitude of 0.07 in the case of sinus mode. In these values of amplitude, the hydrogen recovery was enhanced by a factor of 1.20 and 1.19, respectively. From these results, it is found that the modulation of S/C ratio is favorable for the improvement of methane conversion,however, from the point of view of hydrogen recovery,there is an optimal amplitude for the maximum hydrogen recovery. At this optimum point, the H2/CO ratio was reduced by a factor of 0.95 and 0.93, respectively.This behavior may be explained by the fact that the rate of reforming reaction (CH4+H2O=CO+3H2)has a non-monotonic dependence on water partial pressure, while the dependence of the global reaction rate (CH4+2H2O=CO2+4H2) on water partial pressure is a monotonic function. This means that there would be an optimum amplitude of inlet steam to methane ratio that gives a maximum performance in terms of hydrogen recovery and simultaneously a minimum H2/CO ratio.
In order to shift the equilibrium to the direction of hydrogen production and to accelerate the hydrogen pumping through the membrane, only the sweeping gas was forced. Results obtained for the two modes of modulation (Fig. 3) show that there is a low enhancement of hydrogen recovery only at low amplitudes,but there is no improvement of methane conversion and high H2/CO ratios are obtained. This can be explained by the fact that the hydrogen produced reacts in the opposite reaction direction. It is apparent that the water gas shift reaction plays an important role in determining the reactor performance.
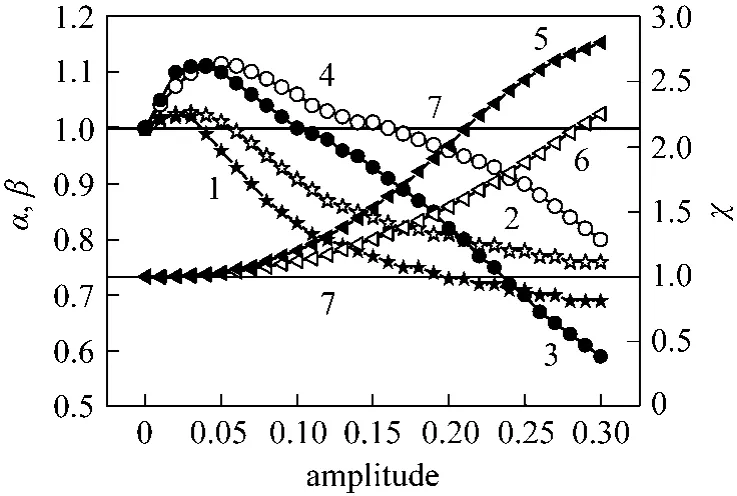
Figure 3 Effect of sweeping gas modulation on the reactor performance1, 2—normalized conversion (α) for square and sinus ways;3, 4—normalized hydrogen recovery (β) for square and sinus ways; 5, 6—normalized H2/CO (χ) for square and sinus ways;7—steady-state conditions
To have a global effect of the inputs on the reactor performance, the three inputs at all possible periodic operations were varied (periodic operations with modulation of more than one input). The system was conducted with S/C and O/C ratios being both in phase while the sweeping gas being 180° out of phase.It was found that varying the sweeping gas cyclically but asymmetrically with respect to S/C and O/C ratios has no effect on hydrogen recovery because the hydrogen pumping effect was overturned. The same results were obtained when the S/C ratio was modulated out of phase while the O/C ratio and sweeping gas were changed in phase. In the two following cases, the effect of modulation is apparent. When the periodic operation was running with all inputs changing cyclically in phaseviaa square mode, it was found that the maxima of enhancement factors of methane conversion and hydrogen recovery were 1.21 and 1.50 obtained at amplitudes of 0.05 and 0.11, respectively. In this interval of amplitude (0.05-0.11), the H2/CO was reduced by a factor ranging from 0.93 to 0.67 compared to that at steady state conditions. Almost the same performance was obtained at an amplitude of 0.15 in the case of the sinus modulation. Since the achieved performance with the square way was obtained at low amplitudes compared to those obtained when the periodic operation was runningviathe sinus way, it is concluded that the square way is better than the sinus way. This improved performance may be explained by the concordance shown by the input modulation where the S/C and O/C ratios enhance the progress of the reforming reaction and the oxidation reaction respectively. Furthermore, the sweeping gas improves the pumping effect of hydrogen through the membrane (Fig. 4).
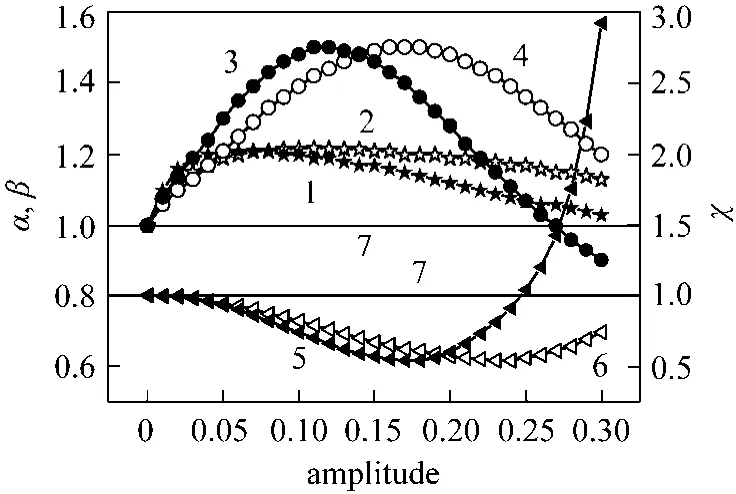
Figure 4 Effect of S/C, O/C and I modulation on the reactor performance (all three inputs in phase)1, 2—normalized conversion (α) for square and sinus ways;3, 4—normalized hydrogen recovery (β) for square and sinus ways; 5, 6—normalized H2/CO (χ) for square and sinus ways;7—steady-state conditions
The hydrogen flux through the palladium membrane depends on the pressure difference between the reaction side and the permeation side. The modulation of the sweeping gas that decreases the partial pressure of hydrogen on the permeation side results in a higher hydrogen separation rate and methane conversion. Thus,the modulation of the sweeping gas ratio is expected to enhance the reactor performance.
When the O/C ratio was varied asymmetrically and the S/C ratio and sweeping gas were in phaseviaa square way, the maximum conversion obtained at an amplitude of 0.1 was 1.20 times higher than that at steady state conditions. However, a higher enhancement factor (1.85) was obtained for hydrogen recovery corresponding to a reduction factor of H2/CO of about 0.66 (Fig. 5). When the modulation was performedviaa sinus way, the following performances were obtained at an amplitude of 0.15: the conversion enhancement factor was only 1.15 times higher than that obtained at steady state conditions, but a higher enhancement factor (1.81) was obtained for hydrogen recovery corresponding to a reduction factor of H2/CO of about 0.67. It is obvious that the S/C ratio favors the hydrogen productionviathe steam reforming reaction and the sweeping gas favors its pumping. The pumping might eliminate the radial distribution of the hydrogen concentration inside the support pores and kept the permeation side under a low pressure, resulting in a relatively high driving force of hydrogen permeation through the palladium membrane. Therefore, the hydrogen flux through the palladium membrane depends on not only the pressure difference between the reaction side and the permeation side, but also the periodic phenomena.
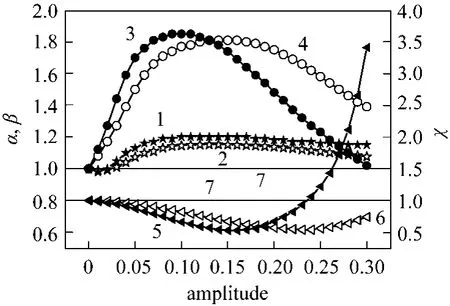
Figure 5 Effect of amplitude modulation on the reactor performance (S/C and I in phase, O/C out of phase)1, 2—normalized conversion (α) for square and sinus ways;3, 4—normalized hydrogen recovery (β) for square and sinus ways; 5, 6—normalized H2/CO (χ) for square and sinus ways;7—steady-state conditions
Because the steady state reaction performance depends on the S/C and O/C ratios, those inputs were modulated in phase at a constant sweeping gas flow rate. It was found that there was not any enhancement of hydrogen recovery but a maximum of methane conversion was obtained at an amplitude of 0.05 and 0.1 for the square and sinus ways, respectively. At a fixed S/C ratio, the modulation of O/C ratio and sweeping gas in phaseviathe square way was applied.The results showed that under this condition, the enhancement of hydrogen recovery was not significant and the maximum obtained at an amplitude of 0.10 yielded an enhancement factor equal to 1.07. At the same amplitude, the conversion of methane was improved by a factor of 1.21. In the case of modulation by the sinus way, it was found that almost the same results were obtained but at an amplitude of 0.15. It is proposed that the periodic operation should be running by switching between the gaseous feed containing O/C=0.5 and S/C=0.5. For this purpose, the asymmetrical cycles were applied in the case of the square and the sinus modulations. The results presented in Fig. 6 show that the maximum of hydrogen recovery achieved in the case of the square modulation was obtained at an amplitude of 0.05 and an enhancement factor equal to 1.33.
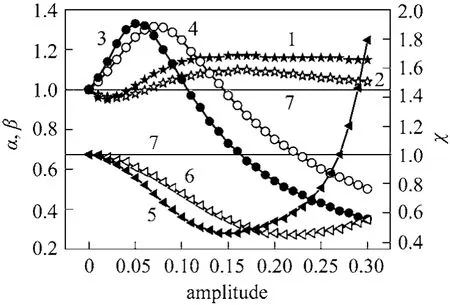
Figure 6 Effect of amplitude modulation on the reactor performance (S/C and O/C ratios via asymmetrical phase)1, 2—normalized conversion (α) for square and sinus ways;3, 4—normalized hydrogen recovery (β) for square and sinus ways; 5, 6—normalized H2/CO (χ) for square and sinus ways;7—steady-state conditions
In general, the conversion of methane was greater than that found under steady state conditions by an enhancement factor of 1.17 at an amplitude of 0.15.The H2/CO ratio corresponding to the achieved maximum hydrogen recovery was reduced to a value of 0.84. When the periodic operation was performed by a sinus function, a lower conversion enhancement factor of 1.09 was achieved at an amplitude of 0.15.The maximum of hydrogen recovery was obtained at an amplitude of 0.07 and an enhancement factor equal to 1.31. The H2/CO ratio was reduced to a value of 0.85 only when the amplitude varied from 0.05 to 0.35.Higher ratios were obtained with further increasing amplitude. It is concluded that operating under periodic operation and switching the input modulation by a square function lead to a better performance.
The results obtained in the case of combination between forcing composition (S/C) and sweeping gas(I) are shown in Fig. 7. It was found that there is a parallel effect between combinations of the modulation of these inputs. Both the maximum conversion and hydrogen recovery were obtained for the same amplitude equal to 0.1 in the case of modulation by a square function and equal to 0.15 for the sinus case.The improvement in conversion and hydrogen recovery can be explained by the pumping effect, which provides efficient hydrogen transport phenomena through the membrane. Then the reaction side was exhausted in hydrogen. In this case, the hydrogen is more produced in the reaction side, and the pumping is provoked by the difference of pressure between the reaction side and permeation side, and by the modulation effect. The equilibrium was shifted towards the direction of hydrogen production, and the conversion of methane increases consequently. It is noteworthy that the enhancement factor of conversion and hydrogen recovery in the case of square modulation are 1.21 and 1.84, respectively while for the sinus mode they are 1.20 and 1.81, respectively. The reduction factor of H2/CO ratio was 0.97 for both cases.
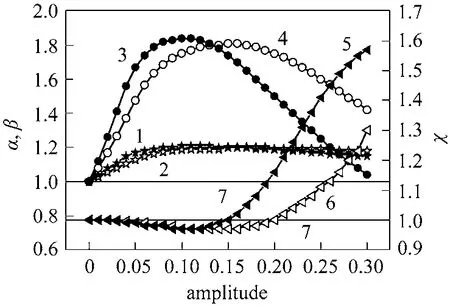
Figure 7 Effect of amplitude modulation on the reactor performance (combination modulation between S/C and I ratios)1, 2—normalized conversion (α) for square and sinus ways;3, 4—normalized hydrogen recovery (β) for square and sinus ways; 5, 6—normalized H2/CO (χ) for square and sinus ways;7—steady-state conditions
The main results obtained in this study are summarized in Table 6. It is noted that these results were obtained only for the amplitudes, which give the best performance in terms of hydrogen recovery. It is obvious that the reactor performed under periodic operationviaa square way leads to a better performance compared to the sinus way modulation.
Because the overage performance was obtained in the case No. 4 with the square way,i.e., the square functions used for modulation of S/C andIchange both in phase while the O/C function is 180° out of phase. The effect of time of modulation was studied at optimal amplitude only for this case. The results show that at the optimal amplitude of 0.1, the reactor performance can be boosted under the forced conditions.Fig. 8 shows that the modulation of these inputs around their steady state values causes a periodic variation in the output parameters (conversion of methane and hydrogen recovery). For example, the hydrogen recovery consists of a repetition of two phenomena: an expansion and a compression, respectively.
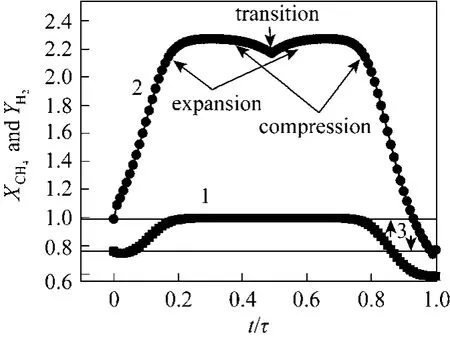
Figure 8 Effect of time modulation by the square way at optimal amplitude (0.1σ=) case when S/C and Iwere in phase and O/C out of phase1—methane conversion ( 4CHX ); 2—hydrogen recovery( 2HY ); 3—steady state conditions
When 0 ≤t/τ≤0.5 or (0≤t≤ π), the two functions used for forcing the steam and sweeping gas are running both in phase. Then, the conversion of methane and the hydrogen produced increases, leading to an increase in the hydrogen recovery. The improvement in methane conversion results from the higher reaction rate due to hydrogen removal from the reaction zone and the positive effect of S/C modulation, which is favourable for the reforming reaction.This is due to the rising edges of the square functions used for modulation of the steam and sweeping gas.This leads to a reduction of hydrogen partial pressure in the permeate side, increasing the driving force for permeation and resulting in the higher rates of hydrogen removal from the reaction zone. It is noted that there is a decrease of hydrogen recovery, which approaches near to 2.2. It seems possible that this fall is due to the falling edges of the two functions used for steam and sweeping gas. Therefore, the part of the function in which the wave goes from high to low values constitutes the first part of the transition band characterized by the compression zone.
At the half timet/τ= 0 .5 or (t=π), there is a half cycle of the square function. At this moment thehydrogen recovery (2HY ) is approximately equal to 2.2. The coordinate (/tτ,2HY )=(0.5, 2.2) is a common point between the end of the compression and the beginning of expansion. This might be explained by the transition band of the square wave caused by the periodic phenomenon, especially the rising and falling edges. Because of the effect dominant of S/C and O/C,the absence of the transition band for the conversion of methane is noted.
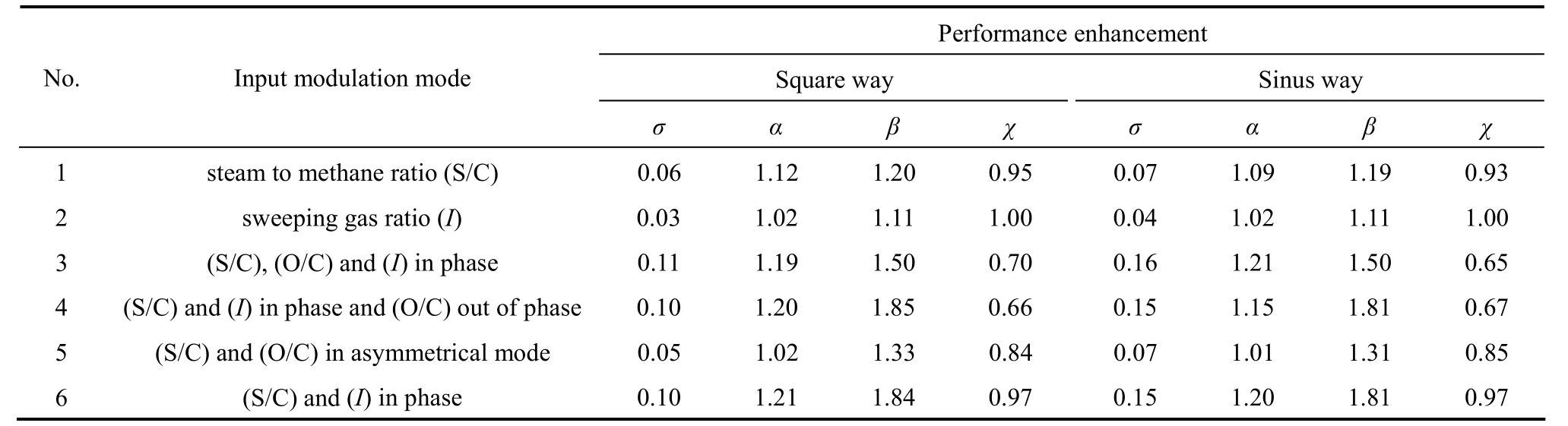
Table 6 Reaction performances for different input modulation
When 0.5/1tτ<< or (2tπ<<π) the second part of the band of transition is observed. There was a change of the polarity from positive to negative values of the two functions used for steam and sweeping gas.On the other hand, the rising edge of the O/C function begins with the positive edge. Then, the hydrogen production is compensated by the reaction of oxidation of methane, which increases the hydrogen partial pressure in the reaction side. The driving force between the reaction and permeation sides guarantees the deficit of the hydrogen pumping from the reaction zone,which is normally provided by the sweeping gas. The end of the transition band is followed by a decrease of hydrogen recovery. This descent (compression) is justified by the fact that the two square functions used respectively for steam and sweeping gas had negative amplitudes. Therefore, the conversion of methane is decreased and the hydrogen produced is reduced, and as a result, the pumping of hydrogen is decreased.
In general, for the complete cycle, it was a gain in hydrogen recovery, which can be explained by the fact that the pumping of hydrogen is stimulated by the effect of periodic operations. The effect of the pressure difference between the reaction and the permeation sides is also involved.
4 CONCLUSIONS
The effect of modulation of inlet composition and sweeping gas on the performance of a Pd-membrane reactor was numerically studied. The numerical investigation has shown that the forced periodic operation can increase conversion and hydrogen recovery if proper operating conditions and forcing are selected.The results obtained in the case of periodic operation were compared among different modulation modes and those of steady state operation. It was found that the periodic operation offered the equivalent performance of the steady state operation with an additional benefit. Furthermore, the variation of the S/C and the sweeping gas ratios via a square way both in phase while the O/C ratio is 180° out of phase at an amplitude of 0.1 could boost the performance, especially in term of pure hydrogen recovery. Under the investigated conditions, the value of the enhancement factor of hydrogen recovery and methane conversion achieved were 1.85 and 1.20 times higher than those found under steady state conditions, respectively. Under the same conditions, the H2/CO ratio is decreased by a reduction factor of 0.66.
The periodic operation is therefore a useful tool to improve the reaction performance. It leads to the assessment of the benefits of periodic operation of a specific reactor system and the identification of the ranges of operating conditions where forced periodic operation is beneficial. This information provides valuable insight for planning detailed experimental studies. Indeed, further studies are required to obtain more detail. This purpose can be achieved using an appropriate equipment for periodic operation such as programmable electronic timers connected to the flow controllers used to generate a periodic function.
ACKNOWLEDGEMENTS
This work was supported in part by the University of Sétif, and the Ministry of Higher Education and Scientific Research (Algeria) with Project No.E01220080023. Both are gratefully acknowledged.
NOMENCLATURE
A reactor section, m2
D diameter, m
ΔEjactivation energy, kJ·mol-1
ΔEphydrogen permeation activation energy, kJ·mol-1
F molar flow, kmol·h-1
ΔH heat of reaction, kJ·mol-1
I sweeping gas ratio
Kispecies adsorption constants
Kjequilibrium constants
kireaction rate constant
L reactor length, m
M molar mass, kg·mol-1
O/C oxygen to methane ratio
P pressure, Pa
Q permeation coeficient of hydrogen, mol·m-1·s-1·Pa-0.5
Q0pre-exponential factor, mol·m-1·s-1·Pa-0.5
R ideal gas constant (=8.314 J·mol-1·K-1)
Rmmembrane radius, m
r reaction rate, kmol·kg-1·h-1
S/C steam to methane ratio
T temperature, K
t time, s
W catalyst mass, g
X conversion
Z dimensionless reactor length
δ membrane thickness, m
η effectiveness factor
ρ catalyst density, kg·m-3
σ amplitude
υ stoichiometric coefficient
ω angular frequency, rad·h-1
Superscripts and Subscripts
forced forced conditions
i reaction species i
j reaction j
p permeation side
r reaction side
ss steady state conditions
0 reactor inlet
1 Dong, H., Shao, Z., Xiong, G., Tong, J., Sheng, S., Yang, W., “Investigation on POM reaction in a new perovskite membrane reactor”,Catal. Today, 67, 3-13 (2001).
2 Lu, H., Tong, J., Cong, Y., Yang, W., “Partial oxidation of methane in Ba0.5Sr0.5Co0.8Fe0.2O3-δmembrane reactor at high pressures”, Catal.Today, 104, 154-159 (2005).
3 Nirmal, G.V., Reddy, B.V., Rosen, M.A., “Feasibility of an energy conversion system in Canada involving large-scale integrated hydrogen production using solid fuels”, Int. J. Hydrogen Energy, 35,4788-4807 (2010).
4 Eisler, M.N., “Getting power to the people: technological dramaturgy and the quest for the electrochemical engine”, History and Technology, 25, 49-68 (2009).
5 Conte, M., Iacobazzy, A., Ronchetti, M., Vellone, R., “Hydrogen economy for a sustainable development: State of the art and technological perspectives”, J. Power Sources, 100, 171-187 (2001).
6 Shu, J., Grandjean, B.P.A., Kaliaguine, S., “Methane steam reforming in asymmetric Pd and Pd-Ag/porous SS membrane reactors”,Appl. Catal. A: Gen., 119, 305-325 (1994).
7 Froment, G.F., “Production of synthesis gas by steam-and CO2-reforming of natural gas”, J. Molec. Catal. A. Chem., 163, 147-156(2000).
8 Chen, Z., Prasad, P., Yan, Y., Elnashaie, S.S.E.H., “Simulation for steam reforming of natural gas with oxygen input in a novel membrane reformer”, Fuel Process. Technol., 83, 235-252 (2003).
9 Chen, Y., Xu, H., Wang, Y., Jin, X., Xiong, G., “Hydrogen production from liquid hydrocarbon fuels for PEMFC application”, Fuel Process. Technol., 87, 971-978 (2006).
10 Chen, Y., Xu, H., Wang, Y., Xiong, G., “Hydrogen production from the steam reforming of liquid hydrocarbons in membrane reactor”,Catal. Today, 118, 136-143 (2006).
11 Houtiet, A., Mahzoul, H., Ehrburger, P., Bernhardt, P., Legare, P.,Garin, F., “Production of hydrogen by steam reforming of methanol over copper-based catalysts: The effect of cesium doping”, Appl.Catal. A: Gen., 306, 22-28 (2006).
12 Kapdan, I.K., Kargi, F., “Bio-hydrogen production from waste materials”, Enzyme Microbiol. Technol., 38, 569-582 (2006).
13 Steinfeld, A., “Solar thermochemical production of hydrogen—A review”, Solar Energy, 78, 603-615 (2005).
14 Basile, A., Paturzo, L., “An experimental study of multilayered composite palladium membrane reactors for partial oxidation of methane to syngas”, Catal. Today, 67, 55-64 (2001).
15 Basile, A., Paturzo, L., Lagana, F., “The partial oxidation of methane to syngas in a palladium membrane reactor: simulation and experimental studies”, Catal. Today, 67, 65-75 (2001).
16 De Groote, A.M., Froment, G.F., “Simulation of the catalytic partial oxidation of methane to synthesis gas”, Appl. Catal. A: Gen., 138,245-264 (1996).
17 Rui, Z., Zhang, K., Li, Y., Lin, Y.S., “Simulation of methane conversion to syngas in a membrane reactor. Part I: A model including product oxidation”, Int. J. Hydrogen Energy, 33, 2246-2253 (2008).18 Christian, E.B., Lodeng, R., Holmen, A., “A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts”, App. Cat. A. Gen., 346,1-27 (2008).
19 Galuszka, J., Pandey, R.N., Ahmed, S., “Methane conversion to syngas in a palladium membrane reactor”, Catal. Today, 46, 83-89(1998).
20 Chen, Y., Wang, Y., Xu, H., Xiong, G., “Efficient production of hydrogen from natural gas steam reforming in palladium membrane reactor”, Appl. Catal. B: Environ., 81, 283-294 (2008).
21 Oklany, J.S., Hou, K., Hughes, R., “A simulative comparison of dense and microporous membrane reactors for the steam reforming of methane”, Appl. Catal. A: Gen., 170, 13-22 (1998).
22 Yu, W., Ohmori, T., Yamamoto, T., Endo, A., Nakaiwa, M., Itoh, N.,“Optimal design and operation of methane steam reforming in a porous ceramic membrane reactor for hydrogen production”, Chem.Eng. Sci., 62, 5627-5631 (2007).
23 Bouwmeester, H.J.M., “Dense ceramic membranes for methane conversion”, Catal. Today, 82, 141-150 (2003).
24 De Falco, M., Di Paola, L., Marrelli, L., Nardella, P., “Simulation of large-scale membrane reformers by two-dimensional model”, Chem.Eng. J., 128, 115-125 (2007).
25 Armor, J.N., “Applications of catalytic inorganic membrane reactors to refinery products”, J. Membr. Sci., 147, 217-233 (1998).
26 Matsumura, Y., Uragami, M., Nakamori, T., Morimoto, S., “A proposal of new hydrogen production process employing equilibrium-shift technology”, J. Jpn. Pet. Inst., 46, 166-171 (2003).
27 Bonrath, W., “Ultrasound supported catalysis”, Ultrasonics Sonochemistry, 12, 103-106 (2005).
28 Silveston, P.L., Hudgins, R.R., “Periodic pressure forcing of catalytic reactions”, Chem. Eng. Sci., 59, 4055-4064 (2004).
29 Silveston, P.L., Hudgins, R.R., Renken, A., “Periodic operation of catalytic reactors—introduction and overview”, Catal. Today, 25,91-112 (1995).
30 Petkovska, M., Nikolic, D., Markovic, A., Seidel, M.A., “Fast evaluation of periodic operation of a heterogeneous reactor based on non linear frequency response analysis”, Chem. Eng. Sci., 65,3632-3637 (2010).
31 Benguerba, Y., Djellouli, B., “Enhancement of the catalytic performances in the case of a consecutive-parallel reaction scheme”, Int.J. Chem. Reactor Eng., 8, A64 (2010).
32 Chan, S.H., Wang, H.M., “Thermodynamic analysis of natural gas fuel processing for fuel cell applications”, Int. J. Hydrogen Energy,25, 441-449 (2000).
33 Tong, J., Matsumura, Y., “Effect of catalytic activity on methane steam reforming in hydrogen-permeable membrane reactor”, Appl.Catal. A: Gen., 286, 226-231 (2005).
34 Ma, L., Trimm, D.L., Jiang, C., “The design and testing of an autothermal reactor for the conversion of light hydrocarbons to hydrogen—I. The kinetics of the catalytic oxidation of light hydrocarbons”, Appl.Catal. A: Gen., 138, 275-283 (1996).
35 Xu, J., Froment, G.F., “Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics”, AIChE J., 35, 88-96 (1989).
36 Trimm, D.L., Lam, C.W., “The combustion of methane on platinum-alumina fiber catalysts—I: kinetics and mechanism”, Chem.Eng. Sci., 35, 1405-1413 (1980).
37 De Smet, C.R.H., De Croon, M.H.J.M., Berger, R.J., Marin, G.B.,Schouten, J.C., “Design of adiabatic fixed-bed reactors for the partial oxidation of methane to synthesis gas. Application to production of methanol and hydrogen-for-fuel-cells”, Chem. Eng. Sci., 56, 4849-4861(2001).
38 Zhang, Y., Xiong, G., Sheng, S., Yang, W., “Deactivation studies over NiO/γ-Al2O3catalyst for partial oxidation of methane to syngas”, Catal. Today, 63, 517-522 (2000).
39 Xiu, G.H., Li, P., Rodrigues, A.E., “Sorption-enhanced reaction process with reactive regeneration”, Chem. Eng. Sci., 57, 3893-3908(2002).
40 Hoang, D.L., Chan, S.H., “Effect of reactor dimensions on the performance of an O2pump integrated partial oxidation reformer: a modeling approach”, Int. J. Hydrogen Energy, 31, 1-12 (2006).
41 Halabi, M.H., De Croon, M.H.J.M., Van der Schaaf, J., Cobden, P.D.,Schouten, J.C., “Modeling and analysis of autothermal reforming of methane to hydrogen in a fixed bed reformer”, Chem. Eng. J., 137,568-578 (2008).
42 Barbieri, G., Brunetti, A., Tricoli, G., Drioli, E., “An innovative configuration of a Pd-based membrane reactor for the production of pure hydrogen: Experimental analysis of water gas shift”, J. Power Sources, 182, 160-167 (2008).
43 Fernandes, F.A.N., Soares, A.B.Jr, “Methane steam reforming modeling in a palladium membrane reactor”, Fuel, 85, 569-573 (2006).
44 Hickman, D.A., Schmidt, L.D., “Synthesis gas formation by direct oxidation of methane over Pt monoliths”, J. Catal., 138, 267-282(1992).
45 Aida, T., Silveston, P.L., Periodic Operation in “Cyclic Separating Reactors”, Blackwell Publishing Ltd., 12-23 (2005).
 Chinese Journal of Chemical Engineering2012年3期
Chinese Journal of Chemical Engineering2012年3期
- Chinese Journal of Chemical Engineering的其它文章
- Experimental and Modelling Studies of Biomass Pyrolysis*
- Synergistic Multilayer Adsorption for Low Concentration Dyestuffs by Biomass
- Kinetics of Photocatalytic Degradation of Gaseous Organic Compounds on Modified TiO2/AC Composite Photocatalyst*
- 3D Numerical Study on Compound Heat Transfer Enhancement of Converging-diverging Tubes Equipped with Twin Twisted Tapes*
- Techno-economic Analysis of Distributed Hydrogen Production from Natural Gas
- Error Analysis of Adsorption Isotherm Models for Acid Dyes onto Bamboo Derived Activated Carbon
