Selective neuronal PTEN deletion: can we take the brakes off of growth without losing control?
Erin A. Gutilla, Oswald Steward,
1 Reeve-Irvine Research Center, University of California Irvine School of Medicine, Irvine, CA, USA2 Department of Anatomy & Neurobiology, University of California Irvine School of Medicine, Irvine, CA, USA3 Department of Neurobiology & Behavior, University of California Irvine School of Medicine, Irvine, CA, USA4 Department of Neurosurgery, University of California Irvine School of Medicine, Irvine, CA, USA5 Center for the Neurobiology of Learning and Memory, University of California Irvine School of Medicine, Irvine, CA, USA
Selective neuronal PTEN deletion: can we take the brakes off of growth without losing control?
Erin A. Gutilla1,2, Oswald Steward1,2,3,4,5,*
1 Reeve-Irvine Research Center, University of California Irvine School of Medicine, Irvine, CA, USA
2 Department of Anatomy & Neurobiology, University of California Irvine School of Medicine, Irvine, CA, USA
3 Department of Neurobiology & Behavior, University of California Irvine School of Medicine, Irvine, CA, USA
4 Department of Neurosurgery, University of California Irvine School of Medicine, Irvine, CA, USA
5 Center for the Neurobiology of Learning and Memory, University of California Irvine School of Medicine, Irvine, CA, USA
How to cite this article: Gutilla EA, Steward O (2016) Selective neuronal PTEN deletion∶ can we take the brakes off of growth without losing control? Neural Regen Res 11(8)∶1201-1203.
Funding: This work was supported by NS073857 to OS, 5T32GM008620 to EG and generous donations from Cure Medical, Research for Cure, and individual donors.
Oswald Steward, Ph.D.,
osteward@uci.edu.
orcid:
0000-0002-8466-9902 (Erin A. Gutilla)
0000-0001-7069-8756 (Oswald Steward)
The limited ability for injured adult axons to regenerate is a major cause for limited functional recovery after injury to the nervous system, motivating numerous efforts to uncover mechanisms capable of enhancing regeneration potential. One promising strategy involves deletion or knockdown of the phosphatase and tensin (PTEN) gene. Conditional genetic deletion of PTEN before, immediately following, or several months after spinal cord injury enables neurons of the corticospinal tract (CST) to regenerate their axons across the lesion, which is accompanied by enhanced recovery of skilled voluntary motor functions mediated by the CST. Although conditional genetic deletion or knockdown of PTEN in neurons enables axon regeneration, PTEN is a well-known tumor suppressor and mutations of the PTEN gene disrupt brain development leading to neurological abnormalities including macrocephaly, seizures, and early mortality. The long-term consequences of manipulating PTEN in the adult nervous system, as would be done for therapeutic intervention after injury, are only now being explored. Here, we summarize evidence indicating that long-term deletion of PTEN in mature neurons does not cause evident pathology; indeed, cortical neurons that have lived without PTEN for over 1 year appear robust and healthy. Studies to date provide only a first look at potential negative consequences of PTEN deletion or knockdown, but the absence of any detectable neuropathology supports guarded optimism that interventions to enable axon regeneration after injury are achievable.
PTEN; mTOR; spinal cord injury; corticospinal tract; motor system; axon regeneration; recovery of function
Introduction
Regeneration of injured or unhealthy axons holds great therapeutic promise for neurological disorders including acute trauma to the brain or spinal cord, stroke, and neurodegenerative diseases. The phosphatase and tensin homolog (PTEN) gene has emerged as an important regulator of axon regeneration, and recent findings by multiple groups support the potential of using PTEN as a therapeutic target. Our recent paper reports that long-term deletion of PTEN, a tumor suppressor gene, does not result in any major detectable pathology and may also enhance neuronal vitality (Gutilla et al., 2016). By selectively deleting PTEN in the motor cortex of young mice, we specifically assessed the effects of PTEN loss using the same approach that has been used to promote regeneration of the corticospinal tract after spinal cord injury.
PTEN’s impact on axon regeneration was discovered in a seminal experiment in which conditional genetic deletion of PTEN promoted robust axonal regeneration of retinal ganglion cells (RGCs) following optic nerve crush (Park et al., 2008). The rationale for this experimental approach is rooted in the discovery of inhibitory intrinsic and extrinsic factors that prevent regeneration of injured axons (Schwab and Bartholdi, 1996; Fitch and Silver, 2008). One approach to overcoming the intrinsic inhibitory factors focuses on attempting to “recapitulate development”, in order to return adult neurons to a more growth permissive state (Filbin, 2006). Growth cessation after the completion of development is thought to occur in part due to the onset of growth inhibiting genes being expressed.
In their landmark study, Park et al. (2008) tested whether axon regeneration could be enhanced if genes that normally repress cell growth were eliminated prior to axon injury. They specifically examined several known tumor suppressor genes including PTEN, p53, retinoblastoma, Smad4, Dicer, and LKB. The effect of each individual gene was studied using multiple strains of “floxed” mice, with each strain having only one of the aforementioned genes flanked by lox-P sites. The specific gene was deleted in the retina be injecting AAV-Cre into the vitreous humor of the eye prior to performing an optic nerve crush. Only deletion of the PTEN gene enabled axotomized RGCs to regenerate, though deletion of both PTEN and p53 reduced retrograde degeneration of RGCs that otherwise occurred. The latter finding, not emphasized at the time, could mean that deleting PTEN enhanced RGC vitality such that the neurons could survive traumatic injuries that would normally cause cell death.
PTEN Deletion and Corticospinal Tract (CST) Axon Regeneration
Following the initial finding that linked PTEN deletion toenhanced neural regeneration, a follow up study tested whether neuronal PTEN deletion could also enhance regeneration after spinal cord injury. This study focused on regeneration of the CST, which mediates voluntary motor function. Damage to CST axons due to spinal cord injury is the cause of paralysis, and enabling regeneration of the CST is the best hope for restoring motor function after injury. Similar to Park et al. (2008), this study used floxed PTEN mice, and PTEN was deleted in the motor cortex of mice one day after birth by injecting AAV-Cre into the sensorimotor cortex. Then, as young adults, mice received spinal cord injuries. Tract tracing of CST axons revealed robust and unprecedented regeneration beyond the injury site (Liu et al., 2010).
Subsequent studies have confirmed and extended findings from these two papers. Genetic deletion of PTEN soon after spinal cord injury in adult mice, or knockdown of PTEN expression using short hairpin RNA (shRNA) against PTEN in adult rats a few days before injury was found to enhance the regenerative growth of the adult CST and recovery of skilled motor functions (Zukor et al., 2013; Lewandowski and Steward, 2014; Danilov and Steward, 2015). Remarkably, PTEN deletion also induced robust CST regeneration in the chronic injury setting one year following injury (Du et al., 2015). These discoveries further highlight the potential of using PTEN interference as a pro-regenerative strategy for treating adult spinal cord injury (Ramon-Cueto et al., 2000).
PTEN's Role in Regulating Normal Neuronal Development and Function
The PTEN gene is thought to exert its growth inhibiting effects through the PTEN protein’s negative regulation of phosphoinositide 3-kinase (PI3K). As a phosphatase, PTEN converts active phosphatidylinositol (3,4,5)-triphosphate (PIP3) to inactive phosphatidylinositol (4,5)-bisphosphate (PIP2), resulting in diminished AKT and downstream mammalian target of rapamycin (mTOR) activation. Thus, deletion of PTEN leads to enhanced levels of PIP3, activation of AKT, and activation of mTOR. The mTOR pathway is well known for its ability to regulate cell growth and proliferation and PTEN’s upstream and non-redundant negative regulation of the mTOR pathway make it a promising pro-regenerative therapeutic target (Don et al., 2012).
It’s here that we come to the theme of our review; is it possible to take the brakes off of such a powerful growth-promoting pathway without losing control? The logic behind testing PTEN was that it had been identified as a tumor suppressor gene. PTEN mutations are common in several cancers, and have been associated with developmental disorders including macrocephaly and autism spectrum disorders (Goffin et al., 2001; Hollander et al., 2011). Experimental studies in which PTEN was deleted during early development in particular cell types revealed neuronal overgrowth, brain enlargement, seizures, and premature death. These studies used mice with a lox-P flanked PTEN gene paired with Cre recombinase expression regulated under the control of promoters including neuron specific enolase (NSE, (Kwon et al., 2006)), glial fibrillary acid protein (GFAP, (Backman et al., 2001; Kwon et al., 2001; Fraser et al., 2004; Yue et al., 2005; Fraser et al., 2008; Wen et al., 2013)), Ca2+/calmodulin-dependent protein kinase II (CamKII, (Sperow et al., 2012)), and the dopamine active transporter (DAT, (Diaz-Ruiz et al., 2009)). In studies using NSE, GFAP, and CamKII promoter driven Cre expression, mice with PTEN deletion exhibit significantly higher postnatal mortality and premature death (~11 weeks of age for CamKII-Cre, (Sperow et al., 2012)). In the GFAPCre models, multiple groups have identified neurons with successful PTEN deletion in the cerebellum, hippocampus, and the cerebral cortex (Backman et al., 2001; Kwon et al., 2001; Fraser et al., 2004, 2008).
The negative consequences following widespread neuronal PTEN loss during development necessitated an in-depth examination of the long-term consequences of deleting PTEN in the way that promotes axon regeneration. As a first step in assessing the potential risk, we employed the same experimental model as in our original report of CST regeneration following spinal cord injury (Liu et al., 2010). PTEN was deleted by injecting AAV-Cre into the sensorimotor cortex of floxed PTEN mice on postnatal day 1. Mice were then allowed to survive for at least one year, and motor function was tested in the final months prior to euthanasia (Gutilla et al., 2016).
Over several months of handling and testing, mice did not exhibit any obvious behavioral abnormalities or spontaneous seizures. General motor function was tested by open field activity and Rotorod, and brains were examined extensively for any evidence of tumors or other neuropathology. Mice with PTEN deletion exhibited normal exploratory activity in an open field and were slightly, though not significantly, impaired on the Rotorod. Most important, we found no evidence of tumors or other neuropathology in the area of PTEN deletion. Cortical motoneurons, the cells of origin of CST axons, appeared healthy and exhibited high levels of immunostaining for the phosphorylated form of ribosomal protein S6 (rpS6). rpS6 phosphorylation is considered a bioindiciator of mTOR activation, so high levels of immunostaining for phosphorylated rpS6 indicates continued activation of mTOR more than a year after PTEN deletion.
The only histological abnormalities were: 1) cortical motoneurons lacking PTEN (identified by retrograde labeling following Fluorogold injections into the spinal cord) were substantially larger than control neurons; 2) there was visible disruption of the normal laminar organization of the cortex in the area of PTEN deletion, perhaps as a result of the increase in neuronal size; 3) the ratio of neuropil to cell bodies was higher in the region of PTEN deletion. Our speculation is that this is due to cellular hypertrophy including hypertrophy of dendrites, but we have not yet assessed this quantitatively.
Safety of PTEN Interference as a Therapeutic Strategy and Remaining Questions
While our study does not qualify as a safety study as would be required for preclinical development of a therapy, the mice survived without any ill effects for up to 18 months after PTEN deletion (considered early old age in mice). Other studies involving hundreds of mice and rats with PTEN deletion in the sensorimotor cortex report enhanced regeneration and improvements in motor function after spinal cord injury, and there have been no reports of negative effects. Taken together, our findings along with previous reports point to the possibility of targeting PTEN therapeutically without triggering untoward effects.
Despite providing an important first look at the long-term consequences of PTEN deletion, several questions remain unaddressed. Other groups have reported seizures following deletion of PTEN early in development (Backman et al., 2001; Ogawa et al., 2007; Pun et al., 2012) as well as changes in the electrophysiological properties of neurons lacking PTEN (Fraser et al., 2008; Sperow et al., 2012; Williams et al., 2015). So far there have been no systematic studies of the physiological consequences of PTEN deletion in cortical neurons, but this warrants further investigation. Additionally, since most acute neurological traumas and neurodegenerative diseases occur in adults, it will also be important to assess the consequences of PTEN deletion in adults.
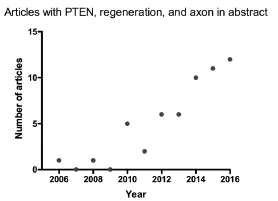
Figure 1 Increased number of articles related to PTEN and regeneration published per year since 2006.
The ability to induce a robust growth capability in central nervous system neurons has broad implications even beyond the potential of enabling regeneration of axons after spinal cord injury. Park’s original study reported that in addition to promoting axon regeneration, PTEN deletion in retinal ganglion cells reduced retrograde cell death following optic nerve crush. It will be of considerable interest to assess whether PTEN deletion could prevent or reverse age-related deterioration of neurons such as neuronal atrophy or even prevent death that is observed in neurodegenerative diseases including Parkinson’s disease, Alzheimer’s disease, and amyotrophic lateral sclerosis (ALS). Indeed, there have been reports that show that deleting PTEN in the substantia nigra reverses symptoms in an experimental Parkinson’s disease model and protects dopaminergic neurons from toxic insults (Diaz-Ruiz et al., 2009; Domanskyi et al., 2011).
The pace of research on PTEN related to neural regeneration is clearly accelerating. A PubMed search done on May 21, 2016 using the keywords “PTEN, regeneration, and axon”in “Abstract” yielded a total of 56 papers, with an increasing number being published each year (Figure 1). Indeed, 12 papers have been published in the first five months of 2016. This increased effort aimed at understanding PTEN’s role in neural regeneration will undoubtedly help to address the questions that still remain.
Author contributions: All authors participated in the organization of the article and the selection of topics to be covered. EAG wrote the majority of the text of the article, edited the figure and figure legend, and provided references. OS provided critical commentary, text, and figure design. All authors approved the final version of the article.
Conflicts of interest: OS is one of the co-founders of the company Axonis, which holds options on patents related to targeting PTEN to enhance regeneration after injury. The terms of this arrangement have been reviewed and approved by the University of California, Irvine in accordance with its conflict of interest policies.
References
Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, Mak TW (2001) Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet 29:396-403.
Danilov CA, Steward O (2015) Conditional genetic deletion of PTEN after a spinal cord injury enhances regenerative growth of CST axons and motor function recovery in mice. Exp Neurol 266:147-160.
Diaz-Ruiz O, Zapata A, Shan L, Zhang Y, Tomac AC, Malik N, de la Cruz F, Backman CM (2009) Selective deletion of PTEN in dopamine neurons leads to trophic effects and adaptation of striatal medium spiny projecting neurons. PLoS One 4:e7027.
Domanskyi A, Geissler C, Vinnikov IA, Alter H, Schober A, Vogt MA, Gass P, Parlato R, Schutz G (2011) Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson’s disease models. FASEB J 25:2898-2910.
Don AS, Tsang CK, Kazdoba TM, D’Arcangelo G, Young W, Zheng XF (2012) Targeting mTOR as a novel therapeutic strategy for traumatic CNS injuries. Drug Discov Today 17:861-868.
Du K, Zheng S, Zhang Q, Li S, Gao X, Wang J, Jiang L, Liu K (2015) Pten deletion promotes regrowth of corticospinal tract axons 1 year after spinal cord injury. J Neurosci 35:9754-9763.
Filbin MT (2006) Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci 361:1565-1574.
Fitch MT, Silver J (2008) CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol 209:294-301.
Fraser MM, Bayazitov IT, Zakharenko SS, Baker SJ (2008) Phosphatase and tensin homolog, deleted on chromosome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience 151:476-488.
Fraser MM, Zhu X, Kwon CH, Uhlmann EJ, Gutmann DH, Baker SJ (2004) Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res 64:7773-7779.
Goffin A, Hoefsloot LH, Bosgoed E, Swillen A, Fryns JP (2001) PTEN mutation in a family with Cowden syndrome and autism. Am J Med Genet 105:521-524.
Gutilla EA, Buyukozturk MM, Steward O (2016) Long-term consequences of conditional genetic deletion of PTEN in the sensorimotor cortex of neonatal mice. Exp Neurol 279:27-39.
Hollander MC, Blumenthal GM, Dennis PA (2011) PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer 11:289-301.
Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ (2001) Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet 29:404-411.
Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF (2006) Pten regulates neuronal arborization and social interaction in mice. Neuron 50:377-388.
Lewandowski G, Steward O (2014) AAVshRNA-mediated suppression of PTEN in adult rats in combination with salmon fibrin administration enables regenerative growth of corticospinal axons and enhances recovery of voluntary motor function after cervical spinal cord injury. J Neurosci 34:9951-9962.
Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z (2010) PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci 13:1075-1081.
Ogawa S, Kwon CH, Zhou J, Koovakkattu D, Parada LF, Sinton CM (2007) A seizure-prone phenotype is associated with altered free-running rhythm in Pten mutant mice. Brain Res 1168:112-123.
Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322:963-966.
Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C, Bronson SL, Murphy BL, Richards DA, Holland KD, Danzer SC (2012) Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron 75:1022-1034.
Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J (2000) Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron 25:425-435.
Schwab ME, Bartholdi D (1996) Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 76:319-370.
Sperow M, Berry RB, Bayazitov IT, Zhu G, Baker SJ, Zakharenko SS (2012) Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration. J Physiol 590:777-792.
Wen Y, Li W, Choudhury GR, He R, Yang T, Liu R, Jin K, Yang SH (2013) Astroglial PTEN loss disrupts neuronal lamination by dysregulating radial glia-guided neuronal migration. Aging Dis 4:113-126.
Williams MR, DeSpenza T, Jr., Li M, Gulledge AT, Luikart BW (2015) Hyperactivity of newborn Pten knock-out neurons results from increased excitatory synaptic drive. J Neurosci 35:943-959.
Yue Q, Groszer M, Gil JS, Berk AJ, Messing A, Wu H, Liu X (2005) PTEN deletion in Bergmann glia leads to premature differentiation and affects laminar organization. Development 132:3281-3291.
Zukor K, Belin S, Wang C, Keelan N, Wang X, He Z (2013) Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J Neurosci 33:15350-15361.
10.4103/1673-5374.189160 Accepted: 2016-08-10
*Correspondence to:
- 中國神經再生研究(英文版)的其它文章
- Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage
- Volume transmission and receptor-receptor interactions in heteroreceptor complexes: understanding the role of new concepts for brain communication
- Uncoupling protein 2 in the glial response to stress: implications for neuroprotection
- TRPV1 may increase the effectiveness of estrogen therapy on neuroprotection and neuroregeneration
- Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery
- Automatic counting of microglial cell activation and its applications

