TGA/Chemometrics addressing innovative preparation strategies for functionalized carbon nanotubes
Roert Risoluti,Giuseppin Gullif,Elen Crcssi,Andre Msotti,Stefno Mterzzi,*
aDepartment of Chemistry,"Sapienza",University of Rome,p.le A.Moro 5,00185,Rome,Italy
bBambino Gesù Children's Hospital,IRCCS,Research Laboratories,v.le di San Paolo 15,00146,Rome,Italy
Keywords:
Carbon nanotubes
Thermogravimetry
Chemometrics
Polyethyleneimine(PEI)
Polyamidoamine dendrimer(PAMAM)
ABSTRACT
In this work,functionalized carbon nanotubes(CNTs)using two polyamine polymers,polyethyleneimine(PEI)and polyamidoamine dendrimer(PAMAM),were investigated by thermal analysis in order to address preparation strategies to obtain low cytotoxic compounds with the ability to conjugate micro-RNAs and,at the same time,to transfect efficiently endothelial cells.Thermogravimetric analysis(TGA)was coupled to chemometrics as a novel analytical strategy to characterize functionalized CNTs from different preparation conditions.In particular,two starting materials were considered:very small CNTs and carboxylated CNTs(CNT-COOH)in order to examine the affinity with polymers.Chemometrics permitted to compare results from TGA and to investigate the effect of a number of factors affecting the synthesis of coated nanotubes including a different amount of involved polymer and the time required for the suspension for a satisfactory and reproducible preparation procedure.The results demonstrated the effectiveness of TGA as a tool able to address synthesis of coated CNTs to be employed as efficient drug delivery vectors in biomedical applications.
1.Introduction
Carbon nanotubes(CNTs)are found to be promising materials in nanomedicine as potential drug carriers,therapeutic agents and diagnostics tools[1].Inparticular,carbon nanotubes possess a good ability to cross cellular membranes because of their nanosize dimension and high surface area[2].In addition,due to their relatively good biocompatibility,CNTs have also been employed as a novel gene delivery system in order to provide personalized medicine[3,4].Despite their attractive features,CNTs in pristine form are not easy to manage because of the solubility[5].Functionalization of them is often required to modify the properties of CNTs by linking radical groups into the surface with the aim of increasing their solubility in organic solvent and in water[6,7].
A number of procedures are proposed for functionalization of nanotubes according to specific applications for which they are prepared[8].Functionalization of CNTs by oxidation,covalent and noncovalent ligand is a procedure currently used for the drug delivery in biomedical field[9—11].
In particular,due to the hydrophobic nature of CNTs,noncovalent functionalization methods are found to be suitable for amphiphilic molecules such as poly(ethylene glycol)(PEG),polyethyleneimine(PEI)or polyamidoamine dendrimer(PAMAM)which show their ability to bind siRNAs or plasmid DNA through electrostatic interactions.All these features result in coated nanoparticles with an excellent transfection efficiency[12,13].Attempts to propose new nanomaterials able to conjugate microRNAs and to efficiently transfect endothelial cells are extensively proposed[14—18]and methods to quickly identify the effectiveness of functionalization are increasingly required.
The investigation of complex matrices is really challenging and innovative approaches aiming at processing multiparametric data are more and more attractive[19,20].In addition,hyphenated techniques and in particular the coupling TGA/chemometrics were recently applied to real complex matrices[21,22]because of the improvement provided by the simultaneousevaluation of more than one variable.In addition,chemometrics associated with thermogravimetric data may be used not only to correlate measurements to one another but also to select the fingerprint range of the TG curves to be used to increase sensitivity and accuracy[23,24].
In this work,the coupling TGA/chemometrics is proposed as an analytical method to characterize coated CNTs to be used as drug delivery vectors for biomedical application.In particular,the multiparametric approach was considered as a tool able to address the most performing conditions of functionalization leading to the most stable product.
2.Materials and methods
2.1.Samples and synthesis
Multi-walled CNTs and CNT-COOH were purchased from Cheap Tubes Inc(Cambridgeport,MA,USA),while PEI(cat no.408727)and PAMAM generation 5(PAMAM G=5,cat no 536709)polymers were purchased from Sigma-Aldrich Co.(St Louis,MO,USA).
Preparation of polyamine-coated CNTs and CNTCOOH was performedusingadispersionofnanotubes(10mg)indifferentsolutions(2 mL)of PEI or PAMAM(40% ,w/w and an exceeding quantity).In addition,the suspensions were placed in a bath sonicator for 30 min and further stirred for 24 h,48 h and 72 h at room temperature.
2.2.Thermogravimetry
All the measurements were performed by a Perkin Elmer TGA7 Thermobalance(Massachusetts,USA).CNTs,polymers and coated nanotubes were placed into the crucible,and temperature was measured using a thermocouple directly attached to the crucible.Regardless to the method,the temperature was raised from 20°C to 800°C,at a heating rate of 10°C/min,as the best resolution rate.An air flow was used as the carrier gas and maintained at a 100 mL/minflow rate.Calibration of the temperature was performed using the Curie-point transition of standard metals in order to ensure an accurate measurement of the sample temperature,as specified by the equipment recommendations.Each sample was analyzed in triplicate and a high reproducibility of the resulting curves was observed.Derivative thermogravimetric data(DTG)were also calculated to compare samples and represent the derivative of the function TG(T)with respect to T.
2.3.Chemometrics
Dataweremathematically pre-tre atedand Principal Component Analysis(PCA)was used as the display method in order to identify directions in the dataset with higher variability.In particular,standard normal variate transform,column autoscaling,and meancentering[25]were considered including the first and second derivatives.Among these,the mean-centering was selected,as it provided the most performing conditions of reaction,ensuring the suitable amount of polymer in each nanotube.Diagnostics and acquisition of the thermogravimetric data were carried out by Pyris software(Thermo Fisher Scientific Inc.,Waltham,MA,USA),while chemometric analysis was performed by Unscrambler X software(CAMO Analytics)to perform statistical analysis.
2.4.Experimental procedure
Thermogravimetric analysis was considered as an analytical tool able to address the synthesis of novel coated carbon nanotubes designed for the delivery of miRNAS,by evaluating the amount of polymer linked to the nanotube.To this aim,a number of variables were considered in this study,including the effect of a different amount of polymer to be linked to the nanotubes(percentage of about 40% and an exceeding amount)and the suitable time of suspension(24 h,48 h and 72 h).In particular,the experimental design consisted of two types of nanotube(CNT and CNT-COOH)and two types of polymer(PEI and PAMAM).The selection of the different conditions of reaction was made on the basis of the research group experience in the field[26,27].The dataset was constructed as follows:for each condition,two levels of concentration and three times of suspensionwereinvestigated in triplicate in order to ensure that the results were not batch-dependent(final dataset of 12×5 data).The most significant variables in samples discriminationwere evaluated by the analysis of the factor loadings and a biplot(plot of the scores and loadings)was used to estimate the variables with the highest squared multiple correlation with the principal components(PCs)and factors affecting most the PCs.
3.Results and discussion
3.1.Thermogravimetric characterization

Fig.1.TG and DTG curves of PEI(A),PAMAM(B),CNT(C)and CNT-COOH(D).
For the two kinds of CNTs and for each preparation,TGA provided the calculation of the percentage of polymer correctly adsorbed onto the nanotube.Preliminarily, the typical thermogravimetric behavior of the two polymers PEI(Fig.1A)and PAMAM(Fig.1B)as well as the two nanotubes CNT(Fig.1C)and CNT-COOH(Fig.1D)was defined as a result of the thermally induced decomposition in the range 20—800°C.
Two main releasing steps may be observed in Fig.1A for PEI corresponding to the thermally induced decomposition of the polymer respectively at 385.9± 0.1°C and 462.9± 0.2°C.Three main releasing steps can be described for PAMAM(Fig.1B):the first is related tothe loss of the residualsolvent while the remaining two derived from the decomposition process of the polymer respectively at 312.9±0.2°C,429.0±0.6°C and 598.6±0.3°C.
Regardless to the nanotubes,the pristine form(Fig.1C)remains stable until 620°C with a decomposition temperature of about 664.4± 0.1°C,while for the carboxylated one(Fig.1D)a slighty decease in the thermal stability may be observed as a consequence of the functionalizationwith a decomposition temperature of about 650,9± 0.1°C.
For each preparation,the resulting TG and DTG curves corresponding to uncoated nanotube(blue),polymer and functionalized nanotube(green)were overlapped and reported in Fig.2,in order to identify different decomposition processes that permit to calculate the percentage of PEI and PAMAM.
With the aim of providing the most performing procedure for coating nanotubes,three main aspects were taken into consideration:the selection of the polymer with the better affinity to the nanotube;the identification of the suitable concentration of polymer between the 40% and an exceeding amount;and the evaluation of the time required for the most performing coating procedure among 24 h,48 h and 72 h.The calculation of the percentage of polymer adsorbed in each coated nanotube provided preliminarly interesting results,as reported in Table 1.
Despite the type of carbon nanotube involved,the PAMAM provided a significantly higher amount of absorbed polymer(P-value of 9.31×10-5and 3.24×10-4respectively for CNT and CNTCOOH).This behavior may be ascribed to the interaction between the hydrophobic part of the polyamine polymer CNTs,leading to the deposition of a layer of polymer onto CNTs.
On the basis of these results,PAMAM was selected as the suitable polymer for the following experiments and the effect of a different amount of starting material(an exceeding quantity)wasevaluated with respect to the percentage of about 40% .Results are reported in Table 2:as the quantity of starting polymer increases,only slighty significant differences may be observed when functionalized CNTs are involved(P-value of 2.73×10-2)while no statistically significant differences are discovered in CNTs-based formulations(P-value of 3.76×10-1).As a consequence,no significant improvement was obtained when a higher amount of polymer was involved,thus suggesting considering the formulation A as the suitable condition for coating processes.

Table 1 Calculated weight losses for coated CNT and CNT-COOH with PEI and PAMAM.

Table 2 Calculated weight losses for coated CNT and CNT-COOH with PAMAM using a percentage of polymer about 40% (Formulation A)and an exceeding amount(Formulation B).
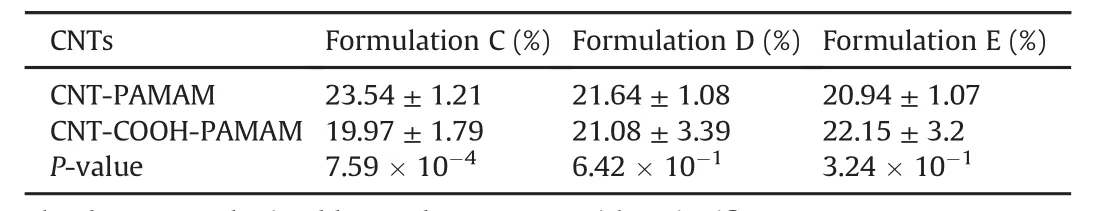
Table 3 Calculated weight losses for coated CNT and CNT-COOH with PAMAM after 24 h(Formulation C),48 h(Formulation D)and 72 h(Formulation E)of suspension.

Fig.2.Overlapped TG and DTG curves of CNT with PEI(A),CNT with PAMAM(B),CNT-COOH with PEI(C)and CNT-COOH with PAMAM(D).
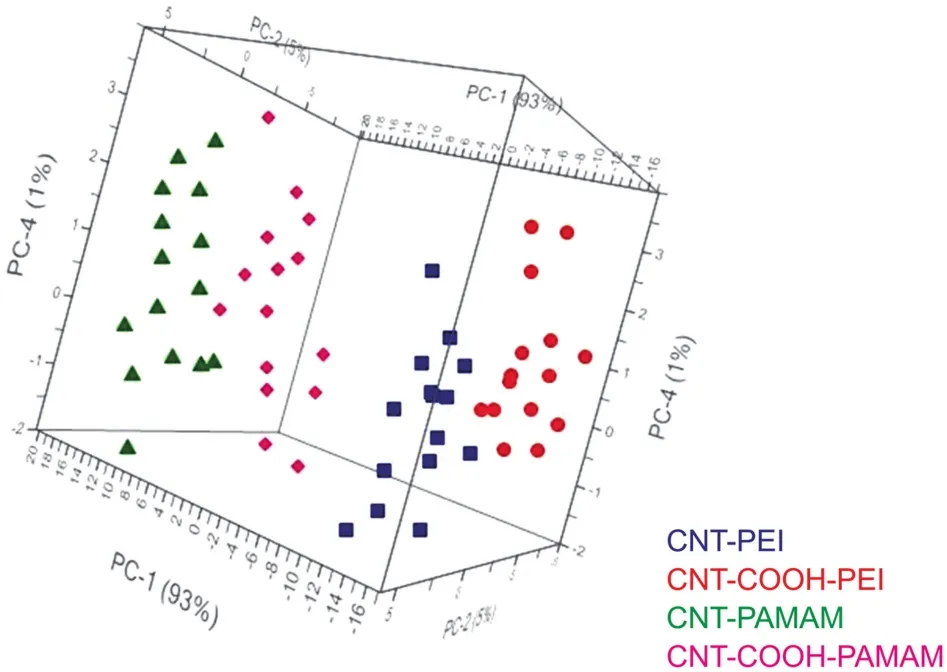
Fig.3.3D-scores plot of samples by principal component analysis(PCA).
The last investigated issue was related tothe optimization of the time of preparation.To this aim,further experiments were planned for both CNT and CNT-COOH considering a time of suspension ranging from 24 h to 72 h.Results are reported in Table 3,where a significantly higher amount of adsorbed polymer was observed for pristine CNTafter 24 h(P-value of 3.76×10-3and 4.46×10-4with respect to 48 h and 72 h,respectively)while results observed for CNT-COOH were not statistically different(P-value of 4.94×10-1and 1.37×10-1with respect to 48 h and 72 h,respectively).As a consequence,the increase in the time of reaction seemed to be not affecting the final amount of PAMAM recovered.
3.2.Multiparametric approach by chemometrics
All the collected data were processed by chemometrics in order to correlate results and to provide a simultaneous evaluation of the investigated variables in a single statistical analysis,and be able to address synthesis to the correct formulation.To this aim,PCA was considered as a simple exploratory tool[28,29]and results are summarized in the 3D-scores plotreported in Fig.3,wheresymbols are used to represent the samples and colours are used for different formulations.
As shown in Fig.3,the mean-centering chemometricfilter permitted to achieve the most performing conditions of reaction,leading to a good reproducibility among measurements as samples belonging to the same preparation(reported in different colours)werefoundtobewellgrouped.Thelocationofthesamplesintheplot suggested that samples seemed to bedifferentiated moving along PC 1(93% ofexplainedvariance)accordingtothepercentage of polymer adsorbed by the nanotubes.In addition,samples resulted further grouped into two main clusters moving along PC 2(5% of explained variance)accordingtothetypeofinvolvednanotube.Thecumulative variance explained by the first four PCs was about 99.8% .
In order to evaluate the contribution of each variable on the location of the samples in the plot,the analysis of the factor loadings was performed and results are summarized in the biplot reported in Fig.4,where symbols are used to represent the scores(the percentage of weight losses)and vectors are involved to locate the loadings(variables)in the plot.
The vector position is the direction with the highest squared multiple correlation with the PCs and indicates the factor affecting most the PCs.Consequently,the interpretation of the biplot provided several important information:first,samples located on the right side of the plot provided the highest value of polymer absorbed by the nanotubes while the samples located on left side of the plot showed the lowest values of weight losses,confirming that PAMAM offers a suitable affinity to natobes with respect to PEI.In addition,the length and the direction of the vectors in the plot suggested that the use of the 40% of polymer provided the most performing outcomes,resulting in a vector affecting most PC 1.Similarly,a time of about 24 h was found to be associated with the samples with higher values of adsorbed polymer as the related vector affected most PC1 with respect to the others.On the contrary,the use of the conditions represented by the vectors “48 h”,“72 h”and“excess”resulted in samples distributed along PC2(5% of explained variance).As a consequence,those vectors slighty affected the samples separation as a function of the amount of polymer linked to the CNTs.
Finally,carboxylated nanotubes provided comparable results as the pristine nanotubes and a significant improvement in the ability of polymer adsorption were observed for pristine CNT with respect to CNT-COOH.All these findings were found to be in accordance with what was observed by the comparison of data by the evaluation of each formulation and demonstrated the ability and the feasibility of the chemometric investigation of TG data to provide the same outcomes in a single multivariate statistical analysis.In addition,the analysis of the factors loadings has clarified the role of each variable permitting to address preparation of polymer coated nanotubes as drug delivery vectors.

Fig.4.Biplot of samples and loadings by principle component analysis(PCA).
4.Conclusions
TGA was considered as an analytical tool able to identify the most performing conditions to obtain coated CNTs for biomedical applications.CNTs demonstrated to be promising materials in nanomedicine.Greater area and strength allow nanotubes to exhibit the properties of chemical and thermal stability.In this work,pristine CNT and carboxylated CNT were compared in order to propose the most performing formulation for drug delivery purposes.Among the investigated conditions,the PAMAM proved to be the suitable polymer allowing to deliver a higher amount of drug.In addition,better results were obtained when the following conditions were observed:a polymer concentration of about 40% and a time of suspension of 24 h.
The association of chemometric tools with TGA permitted a quick identification of little difference in the TG data and demonstrated the capability of estimating the suitable conditions to address the preparation of CNTs.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors thank the Italian Ministry of Health for funding this research(Progetto Ricerca Finalizzata PE-2011-02347026).
 Journal of Pharmaceutical Analysis2020年4期
Journal of Pharmaceutical Analysis2020年4期
- Journal of Pharmaceutical Analysis的其它文章
- Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification
- Polysaccharide-based chromatographic adsorbents for virus purification and viral clearance
- Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants
- Structural elucidation of SARS-CoV-2 vital proteins:Computational methods reveal potential drug candidates against main protease,Nsp12 polymerase and Nsp13 helicase
- Rapid detection of high-risk HPV16 and HPV18 based on microchip electrophoresis
- Solid and liquid state characterization of tetrahydrocurcumin using XRPD,FT-IR,DSC,TGA,LC-MS,GC-MS,and NMR and its biological activities
