Antibio film activity of 3,3’-diindolylmethane on Staphylococcus aureus and its disinfection on common food-contact surfaces
Hui Zhng, Xiomei Guo, Lei Tin, N Wng, Yuqing Li,Ariel Kushmro, Roert Mrks, Qun Sun,*
a College of Biomass Science and Engineering, Sichuan University, Chengdu 610064, China
b Key Laboratory of Bio-resources and Eco-environment of the Ministry of Education, College of Life Sciences, Sichuan University, Chengdu 610064, China
c Key Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, China
d State Key Laboratory of Oral Diseases & National Clinical Research Center for Oral Diseases & Department of Cariology and Endodontics,West China Hospital of Stomatology, Sichuan University, Chengdu 610065, China
e Avram and Stella Goldstein-Goren Department of Biotechnology Engineering, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel
f The Ilse Katz Center for Meso and Nanoscale Science and Technology, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel
Keywords:
Bio film inhibition
3,3’-Diindolylmethane
Extracellular DNA
Extracellular polysaccharide
Food-contact surfaces
A B S T R A C T
This study explored the antibio film efficacy of 3,3’-diindolylmethane (DIM) on Staphylococcus aureus and its disinfection on common food-contact surfaces. The minimum biofilm inhibitory concentration (MBIC)of DIM on S. aureus was 62.5 μmol/L, while it did not impede the bacterial growth evaluated by growth curve and XTT reduction assay. DIM in the concentration range of 31.2-62.5 μmol/L demonstrated a dose-dependent antibiofilm activity to S. aureus, as confirmed by light microscopic (LM), confocal laser scanning microscopic (CLSM), and scanning electron microscopic (SEM) analyses. At DIM of 62.5 μmol/L, the biomass of S. aureus bio film was significantly reduced by 97% and its average thickness by 58% (P < 0.05). DIM of 62.5 μmol/L inhibited the bacterial initial adhesion and proliferation, as well as cell motility; the release of extracellular DNA (eDNA) and extracellular polysaccharide (EPS) were reduced by 75% and 69%, respectively. DIM exhibited a strong inhibition to S. aureus bio film formation on common food-contact surfaces, including 304 stainless steel, glass, and polyvinyl chloride (PVC) but not disperse the mature bio film. Overall, our investigation identified DIM as a promising antibio film agent and its suitability to prevent the bio film formation of S. aureus on common food-contact surfaces utilized during food processing.
1. Introduction
Microb ial bio films are defined as multicellular communities with complex architectures that enable microorganisms to grow and adhere to abiotic or living surfaces [1]. Most o f the pathogens that cause foodborne diseases have capacity to form biofilms on most utensil surfaces and under almost all environmental conditions encountered in food production plants [2]. Bacteria are more likely to form bio films on food-contact surface due to the residue of food ingredients in the food industry [3]. Compared with planktonic bacteria,biofilm-forming cells are more resistant to antimicrobial agents as they have a barrier composed of extracellular polysaccharide (EPS),extracellular DNA (eDNA) and proteins which prevent or reduce the contact with antimicrobial agents [4,5]. When b io films are formed on food-contact surfaces, they are difficult to remove due to the insoluble extracellular matrix (ECM) in which the bacterial cells develop [6,7].There fore, bio films forming on food-contact surfaces made of various materials, such as glass, stainless steel [8]and plastic [9], are often responsible for food contamination and foodbo rne bacterial outbreaks.Hence, how to prevent the formation of bio films is a challenge for the food industry.
Staphylococcus aureusis a commensal and opportunistic pathogen that can form bio film on food and food-contact surfaces, causing food contamination [10]. It was reported that approximately 20%-25% of foodborne bacterial outbreaks were caused byS. aureusin China [11].Bio film formation ofS. aureusis the main cause ofStaphylococcalfoodborne outbreaks. In the food industry, a variety of sanitizers,including benzalkonium chloride, quaternary ammonium compounds,and hydrogen peroxides, have been used to control bio film formation and prevent food contamination [12]. However, the extensive use of sanitizers in the food industry not only failed to completely remove or inactivate pathogens, but has also led to the emergence of resistant bacteria [13-15]. In recent years, resistant bacteria have been found in a variety of foods, such as raw and processed meat [16,17]and dairy products [18]. In order to solve the problems of food contamination caused by biofilm formation and bacterial resistance caused by the wide application of sanitizers, it is urgent to find novel antibiofilm agents that may help to reduce the emergence of bacterial resistance.
Indole (Fig. S1A), a metabolite of tryptophan degradation has been shown to regulate bacterial behaviors such as bio film formation [19],motility [20], virulence [21]and antibiotic resistance [22]. In addition to indole itself, many indole derivatives, including naturally occurring and synthetic ones, have been shown to affect bacterial behaviors.Compared with indole, most of indole derivatives (7-hydroxyindole [23],indole-3-acetaldehyde [24], 3-indolylacetonitrile and indole-3-carboxyaldehyde [25]) have even shown stronger antibio film activity. 3,3’-diindolylmethane (DIM) (Fig. S1B) is the derivative of indol-3-carbinol abundant inBrassicavegetables, which is considered a potent anticancer agent with a variety of biological activities, including anti-inflammatory [26], antiproliferative [27]effectsin vitroand in animal models. Kushmaro et al. [28]reported the antibiofilm activity of DIM against common gram-negative bacteria of medical purpose, however, the effect of DIM on biofilm formation, extracellular matrix closely related to biofilm of gram-positive foodborne bacteriaS. aureus, and its potential application prospect in food industry are still unknown.
Hence, in order to investigate the possible antibiofilm effect of DIM, the present study established the experimental model of theS.aureusbiofilms, an important gram-positive foodborne pathogen.This allowed the evaluation of DIM effect on biofilm biomass,morphology, and the growth cycle of biofilm formation. The effect of DIM on cell motility and extracellular matrix ofS. aureus, as well as the biofilm formed on common food-contact surfaces was also determined.
2. Materials and methods
2.1 Chemicals, bacteria strain, and culture condition
DIM (≥ 98%; CAS: 1968-05-4) was purchased from the Aladdin Industrial Corporation (Shanghai, China) and dissolved in dimethyl sulfoxide (DMSO) (Macklin Biochemical Company, Shanghai, China)to a storage concentration of 2 × 10-2μmol/L. All other chemicals are analytical grade. The strain ofS. aureusused in this study was previously isolated and stored at -80 °C with 25% glycerol in our laboratory. The bacteria were inoculated in tryptone soy broth (TSB)at 37 °C for overnight. Then, overnight cultures ofS. aureuswith optical density value of 0.5 at 600 nm (around 1 × 108CFU/mL)were considered as standard cell suspension used for subsequent experiments.
2.2 Assessment of bio film biomass
The effect of DIM onS. aureusbio film formation was measured in 96-well polystyrene microplates (Corning 3599, USA) as described according to the previously reported method with minor modifications [29-31]. The lowest concentration of DIM, which did not significantly (P> 0.05) inhibit the biofilm formation of the bacteria, was considered as MBIC [32]. In other words, when the concentration reached MBIC, the inhibition effect of biofilm could not be improved even if the concentration was increased. Briefly,5 μL DIM was added individually to each well containing 175 μL brain heart infusion (BHI) broth that was supplemented with 2% glucose,2% sucrose and 2% NaCl in a sterile 96-well polystyrene microplate to reach final concentrations of 7.8, 15.6, 31.2, 62.5 and 100 μmol/L.5 μL of DIM was replaced with 5 μL of DMSO solution as the control. One percent inoculum from standard cell suspension (20 μL diluted standard bacterial suspension in a 1:10 ratio) ofS. aureuswere gently added to each well and incubated at 37 °C for 24 h to form biofilm. After incubation, the planktonic cells were discarded and the wells were washed with 200 μL sterile PBS (Solarbio Science &Technology Company, Beijing, China) for 3 times in order to remove unattached cells. Subsequently, the remaining attached bacteria were fixed with 200 μL of 95% (V/V) methanol solution. After 15 min,the fixatives were discarded and the dried wells were stained with 200 μL of 0.1% crystal violet solution (Solarbio Science &Technology Company, Beijing, China) for 20 min. Then, the staining solutions were discarded and the wells were washed twice with distilled water. After drying, 95% (V/V) ethanol solutions were used to dissolve the stained biofilm for 15 min and the biofilm biomass (optical density value) was quantified using a microplate reader (BIORAD680, USA) at 570 nm. The percentage of inhibition was calculated according to the following formula [33].

2.3 Growth curve analysis
The effect of DIM onS. aureusgrowth was tested as described by Packiavathy et al. [34]. To obtain growth curve, one percent inoculum from standard cell suspension (around 1 × 108CFU/mL) ofS. aureuswere added to 50 mL sterile BHI broth with DIM at 31.2 and 62.5 μmol/L and incubated at 37 °C with 130 r/min for 24 h.The optical density value at 600 nm was measured using UV-visible spectrophotometer (Shimadzu UV-2450, Japan) at every 2 h interval up to 24 h.
2.4 XTT reduction assay
The effect of DIM on the metabolic activity ofS. aureuswas examined using the XTT reduction assay according to the previous method with minor modifications [33]. The sodium salt of XTT and phenazine methosulfate (PMS) was dissolved in PBS at a concentration of 20 and 300 μg/mL, respectively. The biofilm was incubated as described above (method section 2.2).After incubation, the planktonic cells were collected from the 96-well polystyrene microplates. The biofilm cells were washed with sterile PBS for 3 times; the planktonic cells were washed with sterile PBS through repeated centrifugation at 850 ×gfor 5 min and the precipitates were collected. Then, 200 μL of combined solution of XTT and PMS were added to the 96-well polystyrene microplates with washed biofilm and planktonic cells, separately. Following that, 96-well polystyrene microplates were incubated at 37 °C with 130 r/min for 3 h in the dark. Metabolic activity was evaluated by measuring the optical density value at 490 nm using the above microplate reader.
2.5 In situ visualization of S. aureus bio film
2.5.1 Light microscopic (LM) analysis
In order to verify the impact of DIM on theS. aureusbio film, the bio film was investigated microscopically in the presence and absence of DIM as in previous reports [35,36]. Brie fly, the sterile coverslips(φ14 mm) were placed in each well of 24-well polystyrene microplate. 25 μL DIM was added to each well containing 875 μL BHI broth that was supplemented with 2% glucose, 2% sucrose and 2% NaCl in a sterile 24-well polystyrene microplate to reach final concentrations of 31.2 and 62.5 μmol/L. One percent inoculum from standard cell suspension (100 μL diluted standard bacterial suspension in a 1:10 ratio) ofS. aureuswere gently added to each well and incubated at 37 °C for 24 h to allow the formation of bio film. After incubation, the coverslips were washed with sterile PBS for 3 times and stained with 0.1% crystal violet solution for 20 min. Following staining the coverslips were washed with sterile water to remove the excess stain and air dried. Stained coverslips were placed on glass slide and images were captured by a light microscope (Olympus BX53F, Japan) at magnifications of 400×.
2.5.2 Confocal laser scanning microscopic (CLSM) analysis
The effect of DIM on structure ofS. aureusbio film was further analyzed with CLSM (Leica TCS SP5II, Germany) [33,37]. The bio film was grown on the sterile coverslips (φ14 mm) as described the above method 2.5.1. After removal of planktonic cells, biofilm cells were washed with 0.85% NaCl solution and were stained with 3.34 μmol/L SYTO 9 from LIVE/DEAD BacLight bacterial viability kit L7012 (Thermo Fisher Scientific, USA) at 25 °C for 15 min in the dark. The stained biofilm was observed with CLSM.Images were captured using LAS-X software (version 3.3.0; Leica Application Suite X, Leica Microsystems CMS GmbH), and the three-dimensional structure of biofilm was reconstructed using the ImageJ software (version 1.48; National Institutes of Health). In addition, quantitative parameters of the three-dimensional structure were calculated using COMSTAT 2.1 software provided by Dr.Claus Sternberg, DTU Systems Biology, Technical University of Denmark, Denmark.
2.5.3 Scanning electron microscopic (SEM) analysis
To validate the observation obtained with the LM and CLSM,the effect of DIM onS. aureusbiofilm was further examined with SEM (JSM-7500F, Japan) according to the previous method with slightly modifications [38,39]. Brie fly, the sterile coverslips (φ8 mm)were placed in each well of 48-well polystyrene microplate. Then,the bio film was grown on the sterile coverslips (φ8 mm) with DIM at 31.2 and 62.5 μmol/L as described the above section 2.2. Afterwards,the coverslips were washed with PBS for 3 times and immediately fixed with 2.5% glutaraldehyde solution (Leagene Biotechnology Company, Beijing, China) at 4 °C for 3 h. The coverslips were dehydrated in a graded ethanol series of 30%, 50%, 60% 70%, 90%,95% and 100% for 10 min. The samples were then critical-point dried and immediately sputter coated with gold. The bio film was visualized under SEM at magnifications of 2 000× and 5 000×, respectively.
2.6 Growth cycle of S. aureus bio film formation assay
To obtain growth cycle ofS. aureusbiofilm, one percent inoculum from standard cell suspension ofS. aureuswere added to 96-well polystyrene microplates with 200 μL sterile BHI broth that was supplemented with 2% glucose, 2% sucrose and 2% NaCl. After inoculation at 37 °C for different times, the biofilm was quantified with crystal violet staining as described previously at various times up to 120 h. To obtain the effect of DIM on the growth cycle ofS.aureusbiofilm formation, DIM of 62.5 μmol/L was added at different time points of the growth cycle. Then, the biofilm was quantified after 24 h [40].
2.7 Cell motility assay
The motility assays were performed according to the previous reports with minor modifications. Briefly, the plates containing 1% tryptone, 0.25% NaCl and 0.3% agar were prepared for sliding motility [41]. Then, 15 μL of standard cell suspension was inoculated on the center of the plates in the absence or presence of DIM (31.2,62.5 μmol/L) [42]. After incubation at 37 °C for 24 h, the cell motility ofS. aureuswas observed.
2.8 Extracellular matrix measurement
2.8.1 eDNA measurement
The biofilm was grown as described in section 2.5.1 without sterile coverslips, in which eDNA was measured as described by Rice et al. [43]. After discarding planktonic cells and washing the wells, 5 μL EDTA (0.5 mol/L) was added into each well at 4 °C.After 1 h, 700 μL TEN buffer (Leagene Biotechnology Company,Beijing, China) was added into each well to resuspend bio film cells.After centrifugation for 5 min at 4 °C and 18 000 ×g, 100 μL of each supernatant was transferred to a tube containing 300 μL of TE buffer (Solarbio Science & Technology Company, Beijing, China).Then, the mixture and equal volume of binding buffer (Gel extraction kit, OMEGA) was added to adsorption columns. After 2 min at room temperature, eDNA was collected by centrifugation at 4 °C and 18 000 ×gfor 60 s. The adsorption columns were washed with wash buffer (Gel extraction kit, OMEGA) through repeated centrifugation at 1 800 ×gfor 30 s. Finally, after adding sterile water, eDNA was collected by centrifugation. The concentration of eDNA was measured by spectrophotometer (BioDrop BD1110, England).
2.8.2 EPS measurement
EPS in biofilm were determined by colorimetric method of anthrone sulfuric acid [44]. DIM was added to round cell culture dish (φ60 mm) containing 5 mL BHI that was supplemented with 2% glucose, 2% sucrose and 2% NaCl to reach final concentrations of 31.2 and 62.5 μmol/L. Then, 1% inoculum from standard cell suspension ofS. aureuswere added to each dish and incubated at 37 °C for 24 h to allow the formation of biofilm. After discarding planktonic cells, the bio film was gently washed 3 times with sterile PBS to remove unattached cells before suspended in 2 mL of PBS.The bio film cell suspensions were centrifuged at 9 500 ×gfor 10 min.The resulting supernatant and 6 mL anthrone sulfuric acid solution (0.100 g anthrone reagent are soluble in 100 mL 80% sulfuric acid) were mixed thoroughly, then heat mixture in boiling water at 95 °C for 15 min, and cool mixture in ice water for 15 min. The optical density value at 625 nm were recorded, and the concentration of EPS was calculated according to the standard glucose curve.
2.9 Antibio film activity of DIM on common food-contact surfaces
Biofilm was grown on the sterile 304 stainless steel, glass and PVC pieces (φ8 mm) placed in 48-well polystyrene microplates without and with DIM (15.6, 31.2 and 62.5 μmol/L) as described the above section 2.2. After incubation, the 304 stainless steel,glass and PVC pieces were washed with sterile PBS for 3 times and stained with crystal violet as described in section 2.2. The biofilm biomass (optical density value) was quantified using the above microplate reader at 570 nm. In addition, the number of active cells in bio film was determined by plate counting method. Brie fly, after washing the pieces, the bio film was resuspended with 1 mL PBS. The suspensions that were diluted with 1:10, 1:100 and 1:1 000 was spread TSB solid plate and the count was recorded after incubating at 37 °C for 24 h.
2.10 Mature Biofilm disruption assay on common foodcontact surfaces
Mature bio film was grown on the sterile 304 stainless steel, glass and PVC pieces (φ8 mm) placed in 48-well polystyrene microplates without DIM. After incubation, the 304 stainless steel glass and PVC pieces were washed with sterile PBS for 3 times, then mature bio film were treated with DIM (15.6, 31.2 and 62.5 μmol/L) and positive control. NaOH (10 g/L) and HNO3(10 g/L) solution were adopted as positive control. After 15, 30 and 45 min of treatment, biofilm was stained as described the above section 2.2. The bio film biomass (optical density value) was quantified using microplate reader at 570 nm.
2.11 Statistical analysis
All experiments were performed in triplicate at least three times.All data were expressed as arithmetic mean ± standard deviation and analyzed with Dunnett-ANOVA test using the SPSS software (version 20.0; SPSS, I., USA). A probability value atP< 0.05 was considered significant.
3. Results
3.1 Dose-dependent inhibition of biofilm formation in S. aureus by DIM
The antibiofilm activity of DIM was assessed by measuring the binding of crystal violet to biofilm cells ofS. aureuson 96-well polystyrene microplate. A concentration dependent increase in antibiofilm activity was observed (Fig. 1). DIM at 31.2 and 62.5 μmol/L showed 50% and 90% antibio film activity, respectively.No significant increase in the antibiofilm activity was observed at higher concentrations than 62.5 μmol/L (P> 0.05). Thus 62.5 μmol/L was considered as MBIC.

Fig. 1 The effect of DIM on bio film formation of S. aureus. Data were presented as the mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001.
3.2 Non-fatal effect of DIM on S. aureus growth
The effects on bacterial growth and biofilm structure are the two important criteria to determine the efficacy of any antibiofilm agent [32]. The promising antibio film agents have the characteristics of having little or no effect on bacterial growth under conditions of high antibiofilm activity [45]. In order to explore whether the high inhibition effect of DIM on bio film was realized by inhibiting the growth, the concentrations with at least 50% antibiofilm activity (MBIC and 1/2 MBIC) were selected to study the effect onS. aureusgrowth. After 24 h of incubation, no significant differences were observed in the cell densities between control and DIM (31.2 and 62.5 μmol/L) treated (Fig. 2A). Further, DIM have no antibacterial activity as con firmed by quantifying and comparing the total number of metabolically active cells in the control and treated wells using XTT reduction assay. The number of metabolically active cells in the bio film cells was reduced in a dose-dependent manner and the number of metabolically active cells in planktonic cells was found to increase in a dose-dependent manner when treated with DIM from 31.2 μmol/L to 62.5 μmol/L (Fig. 2B). On the other hand, no significant difference was detected among the total metabolically active cells in control and DIM-treated-samples (P> 0.05). This con firmed that DIM has no antibacterial activity againstS. aureusat tested concentrations.
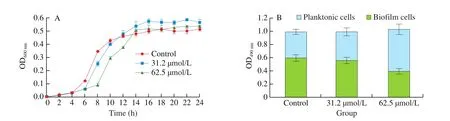
Fig. 2 The effect of DIM on growth of S. aureus. (A) A graph depicting the OD600 nm of S. aureus grown in the presence and absence of DIM. (B) The viability of planktonic and bio film cells of S. aureus were determined separately by XTT assay. Control: dimethyl sulfoxide. Data were presented as the mean ± S.D.
3.3 In situ visualization of S. aureus bio film
3.3.1 LM and CLSM analysis
The effect on bacterial growth and biofilm architecture are the two important criteria to determine the efficacy of antibio film agents.The LM and CLSM images clearly revealed the morphology ofS. aureusbiofilm on the coverslips after DIM treatment. The untreated coverslips showed a clumping of complex bio film, whereas a remarkable reduction in biofilm formation was observed on the DIM-treated-coverslips (Fig. 3). Moreover, the bio film covered surface area and biofilm thickness in the presence of DIM were reduced in a dose-dependent manner (Fig. 3B). Further, the three-dimensional images analysis using COMSTAT 2.1 software showed a reduction in the thickness ofS. aureusbio film on the coverslips in the presence of DIM. The biomass and thickness of the bio film was reduced in a dose-dependent manner when treated with DIM from 31.2 μmol/L to 62.5 μmol/L. The biomass ofS. aureusbiofilm was significantly reduced by 30% when exposed to 31.2 μmol/L DIM but by 97%,when exposed to 62.5 μmol/L (P< 0.05). The thickness ofS. aureusbio film was significantly reduced by 34%, and by 58% when exposed to 31.2, 62.5 μmol/L of DIM, respectively (P< 0.05) (Table 1). These images and quantitative analysis suggested that DIM had the ability to inhibit the formation ofS. aureusbio film.

Fig. 3 Microscopic visualization of antibio film efficacy of DIM on S. aureus. (A) Light microscopic images (400×). (B) Confocal laser scanning microscope images (400×) in the presence and absence of DIM.

Table 1Inhibitory effect of DIM on the formation of S. aureus bio film.
3.3.2 SEM analysis
SEM analysis was also carried out to confirm the antibiofilm potential of DIM on formation ofS. aureusbio film. The bio film of untreated control was observed to adhere on the surface of coverslips and form thick aggregates, while the biofilm exposed to DIM gradually decreased. As shown in Fig. 4, the biofilm treated with 31.2 μmol/L DIM showed slackened and small cell clusters. And the biofilm in the presence of higher concentration of DIM (62.5 μmol/L)were of dispersed colonies of fewer cells and attached loosely on the glass coverslips. Moreover, extracellular matrix (the red arrow) was clearly visible in the untreated control group, but not in the treatment group.
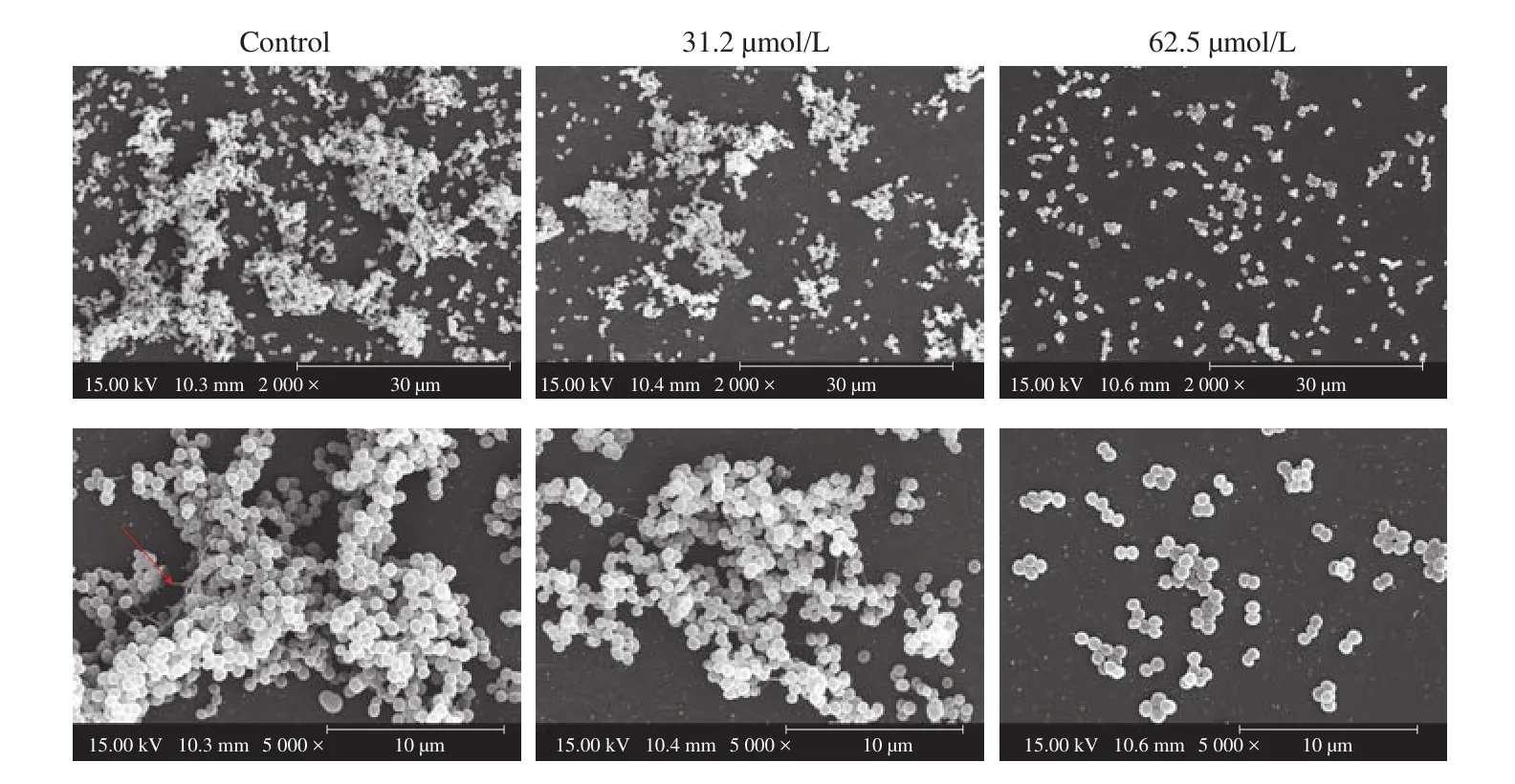
Fig. 4 Scanning electron micrographs of S. aureus bio film formed on the coverslips in the presence and absence of DIM. Images were taken at 2 000× and 5 000× magnification. Red arrow, extracellular matrix.
3.4 Effect of DIM on the growth cycle of S. aureus bio film
Biofilm formation generally divided into several steps: initial attachment, bacterial aggregation, maturation and dispersion [46]. The effect of DIM on the growth cycle ofS. aureusbio film is shown in Fig. 5. The growth cycle ofS. aureusbio film was measured and the results showed that between 0 and 3 h there was the bacterial initial adhesion stage, between 3 and 8 h was the bacterial aggregation stage occurred, 8-48 h was the biofilm maturation stage and the biofilm diffusion stage occurred after 48 h (Fig. 5A). To explore which phase of the growth cycle ofS. aureusbiofilm DIM influenced, DIM of 62.5 μmol/L was added to the cultures at different time points during the growth cycle ofS. aureusbio film. Addition of DIM immediately (0 h)after inoculation resulted in 90% inhibition of biofilm formation (Fig. 5B). DIM inhibited the biofilm obviously when added at 2, 4 and 6 h after inoculation during the adhesion and proliferation stages. Though, 8 h following inoculation during the growth phase, the bio film cultures were resistant to DIM (Fig. 5B).These results demonstrated that DIM interfered with the formation ofS. aureusbio film when added during the bacterial initial adhesion and proliferation stage.
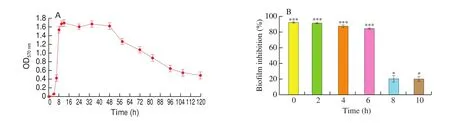
Fig. 5 (A) The growth cycle of S. aureus bio film. (B) The effect of DIM on the S. aureus bio film after adding at different time points in growth cycle of S. aureus bio film. Data were presented as the mean ± S.D. *P < 0.05, ***P < 0.001.
3.5 Effect of DIM on the cell motility of S. aureus
During the initial bacterial adhesion stage, motility plays a crucial role in adhesion to surface and to the subsequent bio film formation [41].S. aureusis a non-flagellated bacterium with a motility defined as sliding or colony spreading. Hence, the ability to interfere with the sliding ofS. aureusof DIM was tested. Fig. 6 showed a dose-dependent attenuation of sliding with DIM treated in the concentration range from 31.2 μmol/L to 62.5 μmol/L. The maximum inhibition of sliding was observed at 62.5 μmol/L.

Fig. 6 The effect of DIM on sliding of S. aureus. Control: dimethyl sulfoxide.
3.6 Effect of DIM on the release of eDNA and EPS of S. aureus
ECM is a key factor in the formation of the three-dimensional structure of biofilm [47]. eDNA and EPS play important roles in the initial adhesion and the proliferation stage of bacteria,respectively [48,49]. Therefore, we examined the inhibitory ability of DIM on the release of eDNA and EPS. The concentration of eDNA and EPS inS. aureusbiofilm are given in Fig. 7. A significantly decrease of eDNA and EPS was observed in a dose-dependent manner with DIM treated in the concentration range from 31.2μmol/L to 62.5 μmol/L (P< 0.05). At the concentration of 62.5 μmol/L, the eDNA and EPS content in the extracellular matrix was 1.7 and 11.6 μg/mL.They were inhibited by 75% and 69%, respectively.
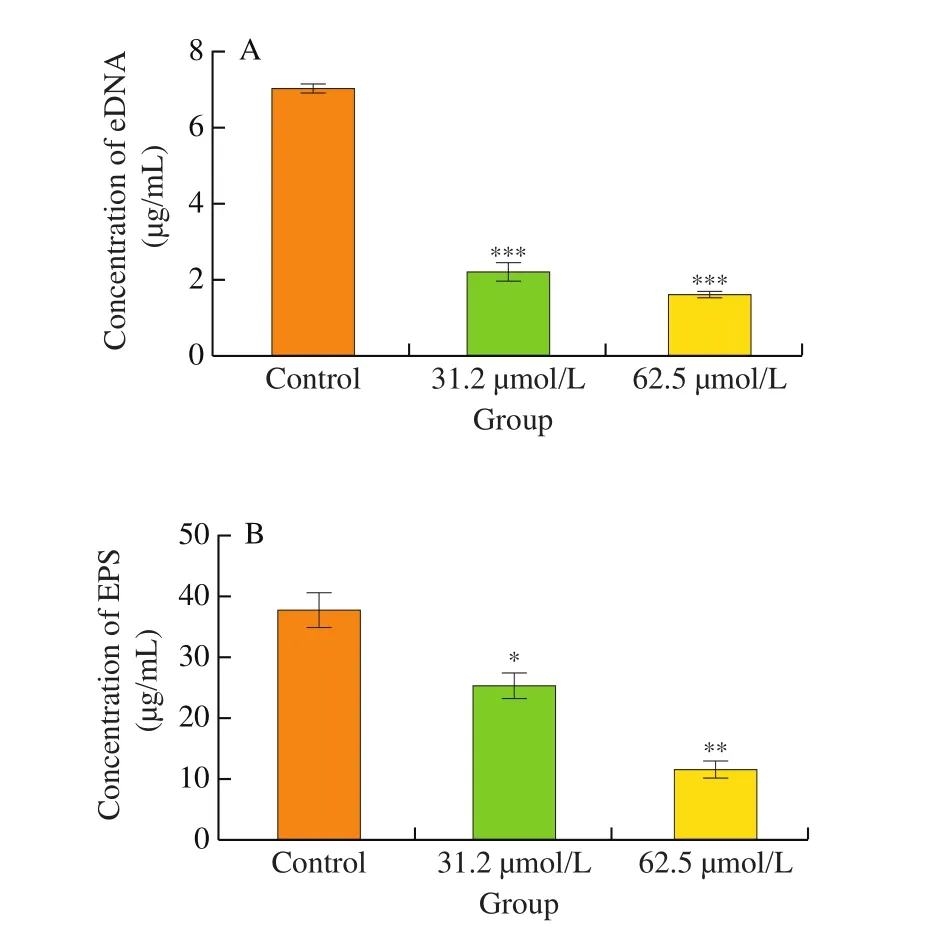
Fig. 7 (A) The effect of DIM on the release of eDNA. (B) The effect of DIM on the release of EPS. Control: dimethyl sulfoxide. Data were presented as the mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001.
3.7 Effect of DIM on biofilm formation of S. aureus on common food-contact surfaces
The surface property of the carrier is also a crucial factor affecting the attachment and bio film formation [50,51]. Hence, we investigated whether DIM inhibit the bio film formation ofS. aureuson common food-contact surfaces. Compared with PVC and glass,S. aureusis more likely to form bio film on 304 stainless steel surfaces (Fig. 8A).The ability of DIM to inhibit the formation ofS. aureusbio film on 304 stainless steel, glass and PVC was determined. Fig. S2 shows a dose-dependent increase of bio film inhibition rate with DIM treated in the concentration range from 15.6 μmol/L to 62.5 μmol/L. DIM at 62.5 μmol/L had better antibio film activity on 304 stainless steel and glass surface, and the bio film inhibition rate was nearly 80%. As can be seen from Fig. 8B, the number of viable cells in the bio film formed on 304 stainless steel was the highest, con firming thatS. aureuswas more likely to form biofilm on 304 stainless steel. A significantly decrease of viable cells was observed in a dose-dependent manner with DIM treated in the concentration range from 15.6 μmol/L to 62.5 μmol/L (P< 0.05) (Fig. 8B). Approximately 4.4 × 106CFU/coupon ofS. aureusin the biofilm on 304 stainless steel was detected in the absence of DIM, whereas 3.0 × 105CFU/coupon ofS. aureuswas observed in the presence of DIM (62.5 μmol/L) (Fig. 8B).The results showed that DIM was able to inhibit the formation ofS. aureusbiofilm on common food-contact surfaces such as glass,PVC, and especially 304 stainless steel that was the most commonly utilized in food industry.

Fig. 8 (A) The ability of bio film formation by S. aureus on common food-contact surfaces. (B) The effect of DIM on the viable cells in the S. aureus bio film. Control: dimethyl sulfoxide. Data were presented as the mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001.
3.8 Effect of DIM on mature bio films of S. aureus on common food-contact surfaces
When evaluating the mature biofilm disruption ability of DIM onS. aureuson common food-contact surfaces, the crystal violet quantification showed that DIM did not exhibit any significant disruption on mature bio films ofS. aureus(Table S1). DIM failed to demolish mature bio film ofS. aureusat all tested concentrations.
4. Discussion
S. aureusis one of the leading foodborne pathogens that cause foodborne outbreaks worldwide [52].S. aureuscan form biofilm on food and food-contact surfaces by adherence, colonization, and development of extracellular matrix, thereby affecting the quality of food. Importantly, biofilm is difficult to completely remove once formed, and resistant bacteria are increasing due to the wide application of sanitizers in the food industry. Hence, the current study focused on an antibiofilm agent that could effectively inhibit the bio film formation ofS. aureuswithout exerting any selection pressure over the growth. Here we reported the antibio film activity of DIM on the foodborne pathogenS. aureus. The antibio film activity of DIM at MBIC was up to 90% in 96-well polystyrene microplates by crystal violet staining assay, which was higher than those by most small molecule compounds reported so far [53-55]. In addition, the results of growth curve and XTT reduction assay revealed its inefficiency in killingS. aureuscells. Thus, it is appropriate to state that the antibiofilm potential of DIM at MBIC is not due to its inhibitory effect on bacterial growth.
The most prominent feature of a bio film is the three-dimensional structure formed by colony aggregation and stacking [56]. The micrographs of CLSM demonstrated that DIM imposed a collapse of the bio film architecture ofS. aureusby loosening its microcolonies.Furthermore, the substantial reduction in biomass and thickness of bio film treatment with DIM was demonstrated through COMSTAT 2.1 software analysis. Likewise, SEM analysis con firmed the remarkable antibiofilm efficiency of DIM. These results suggested that DIM prevented the attachment of the bacterial cells and the formation of three-dimensional architecture resulting in a failure in the formation of a biofilm. This is in accordance with the previous reports that many antibiofilm agents were able to disrupt the complex biofilm architecture and slacken microcolonies [57-59].
The planktonic cells aggregated to form a complex three-dimensional structure of biofilm through a variety of physiological factors and exhibited different physiological activities such as cell motility, initial attachment, cell proliferation and production of ECM at different stages [49]. We found that DIM terminated bio film growth ofS. aureuswhen it was added at the initial adhesion and proliferation stage. This may have been due to its effect on bacterial motility as it was reported that motility was related to the adhesion of cells to abiotic surfaces and that played an important role in bio film formation [60]. Indeed, our study revealed that DIM could inhibit the sliding ofS. aureus. Kaito et al. [61]and Fuente-Nú?ez et al. [62]reported the factors influenced in motility could inhibit the efficiency of bio film formation.
S. aureusbio film is often encased in a self-produced extracellular matrix which is the major reason for the three-dimensional structure of bio film [63]. eDNA is an essential matrix molecule, and previous reports suggested the presence of eDNA on bacterial cell surface enhances adhesion and surface aggregation due to the involvement of acid-base interactions [48,64,65]. We measured the content of eDNA in the bio film and the results demonstrated DIM also inhibited the eDNA release. As previously reported, antibiofilm agents may impede the formation ofS. aureusbio film by reducing the expression ofcidA, a murein hydrolase regulator, to inhibit eDNA release [66].EPS, a major contributor to the structural integrity of biofilm, is produced mainly during the proliferation stage [48]. Previous reports showed the ability of potent antibio film to reduce the EPS content inS. aureusbiofilm [67,68]. A similar phenomenon was observed in our study demonstrating that DIM could prevent the production of EPS byS. aureus. These results suggested that DIM might inhibit the formation ofS. aureusbio film by inhibiting the release of eDNA and EPS, thus damaging the integrity of three-dimensional structure. This is supported by the SEM images showing that the ECM around the bio film cells decreased with increasing DIM concentration.
Although the majority of planktonic cells are susceptible to common antimicrobials, mature biofilms with ECM are usually resistant to antimicrobials [69], thus, it is important to remove previously established bio films. Similar to multiple previous studies of testing small molecule compounds, DIM failed to disrupt mature bio film ofS. aureusat all concentrations tested [33,35]. Because ECM is formed by the aggregation of proteins, eDNA, polysaccharides and other macromolecules, the breaking down of a mature bio film may be largely depending on the chemical processes including acid/base (e.g.,HNO3, NaOH, etc.) effects, or enzymatic processes, such as proteases,nucleases, lysostaphin and glycogen hydrolases, etc. [70].
In the food industry, biofilm formed on food-contact surfaces has shown to be a major cause of food contamination by a variety of investigations [71,72]. We found thatS. aureushad the greatest biofilm formation on 304 stainless steel among the three-common food-contact surfaces tested. Interestingly, DIM was shown to reduce the biofilm formation ofS. aureuson 304 stainless steel by nearly 80% and the viable cells in biofilm by 90% in our investigation.Indeed, the inhibitory effect of antibiofilm agents on the biofilm formation ofS. aureushas been reported in multiple previous studies [35,54,73], but most of them were based on 96-well polystyrene microplates analysis and few had evaluated their effects on food-contact surface commonly used in food industry like 304 stainless steel. Rodriguesde et al. [74]did report the effect of carvacrol on the biofilm formation ofS. aureuson both 96-well polystyrene microplates and 304 stainless steel surfaces, however, carvacrol could only effectively inhibit the biofilm on 96-well polystyrene microplates but not on stainless steel surfaces, neither to the viable cells (reduced by around 15% after 72 h). These results suggested that DIM exert strong inhibition activity against the formation ofS. aureusbiofilm on 304 stainless steel, the representing food-contact surface in food industry.
Additionally, animal and pharmacokinetic experiments showed that DIM was low in toxicity—it was well tolerated in healthy subjects at single doses of up to 200 mg (0.8 mmol) [75,76], which is 12 times higher than the highest concentration used in the present sutdy,and DIM had high stability under both acidic and high temperature conditions, which is common during food processing [77,78]. Thus,DIM has a good application prospect in the food industry. Since foodborne pathogens may still be remained on food-contact surfaces at small quantity thus to form colony and even bio films, especially in hard-to-clean areas, such as cracks, gaskets, and pipe joints, despite the routine cleaning, sanitation and disinfection applied during food processing. DIM mainly hindered the bacterial adhesion and colony formation to prevent the biofilm formation, thus it is suitable to be used to wash and temporarily stay on the food-contact surface after conventional cleaning and disinfection to prevent the biofilm formation. Of course, at present the inhibition effect of DIM on bio film formation is preliminarily investigated in the laboratory, and the process parameters of its application in the food industry need to be further determined.
5. Conclusion
The antibio film activity of DIM onS. aureuswas evaluated for the first time and our investigation demonstrated that DIM could be applied to inhibit the formation ofS. aureusbio film on common food-contact surfaces. Since DIM had little effect on bacterial growth even at the concentration of effective biofilm inhibition, it has the potential to be developed into a novel antibio film agent to prevent the bio film formation of common foodborne pathogens likeS. aureuson food-contact surfaces thus ensuring food safety.
Declaration of competing interest
The authors declared that there was no conflict of interest.
Acknowledgments
This work was supported by the National Key Research and Development Projects (2019YFE0103800); Sichuan Science and Technology Program (2019YFH0113, 2021YFH0060,2021YFH0072, 2021ZHFP0045, 2021YFN0092); and the Fundamental Research Funds for the Central Universities(2018CDLZ-07, 2018CDPZH-9, 2019CDPZH-23, 2020CDLZ-17).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.04.017.
- 食品科學與人類健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species

