Charactering the spoilage mechanism of “three sticks” of Jinhua ham
Changyu Zhou, Guang Zhan, Daodong Pan, Guanghong Zhou,Ying Wang*, Jun He, Jinxuan Cao,*
a State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products, Ningbo University, Ningbo 315211, China
b Key Laboratory of Animal Protein Food Processing Technology of Zhejiang Province, Ningbo University, Ningbo 315211, China
c Key Laboratory of Meat Processing and Quality Control, MOE; Key Laboratory of Meat Processing, MOA; Jiangsu Synergetic Innovation Center of Meat Processing and Quality Control; Nanjing Agricultural University, Nanjing 210095, China
Keywords:
Jinhua ham
Three sticks
Spoilage
Microbial counts
Volatile compounds
Biogenic amine
A B S T R A C T
To investigate the spoilage characteristics of Jinhua ham, sensory scores, volatile compounds, biogenic amine,physicochemical parameters and microbial counts were evaluated between normal and spoiled hams. The results showed that off-odors of spoiled hams were dominated by rancid, sour, sul fide and ammonia odors derived from these compounds including butanoic acid, methanethiol and dimethyl disul fide. Total content of biogenic amine in spoiled hams was significantly higher (more than 10-fold) compared with normal hams, and putrescine, cadaverine and histamine were the key components of biogenic amine of spoiled hams. Lower salt content, and higher moisture, TVB-N and thiobarbituric acid reactive substances (TBARS)values were observed in spoiled hams compared with normal hams. The populations of Enterobacteriaceae and Enterococcus of spoiled hams were obviously higher than that of normal hams. High moisture and low salt content caused the abnormal growth of Enterobacteriaceae and Enterococcus in spoiled hams, which contributed to the spoilage of Jinhua ham.
1. Introduction
Jinhua ham is one of the traditional dry-cured meat products in China, which is produced through a long period of more than 8 months and well received by consumers due to its distinct flavor [1].The traditional procedure of Jinhua ham consists of multiple stages including raw ham preparation, salting, washing, sun-drying, shaping,ripening and post-ripening [2]. The raw materials of traditional Jinhua ham are from the hind legs of the Liangtouwu, which has characteristics of low growth rate and fertility [3]. In contrast,Landrace pigs, a commercial breed of Danish origin, show rapid growth rate and high carcass yield [4]. The weight of each hind leg is more than 13 kg. As a result, Landrace pigs are considered to be the main raw material for the processing of modern-processed Jinhua ham. Modern processes of Jinhua ham included raw ham preparation,salting, washing, low-temperature dehydration, moderate-temperature fermentation and high-temperature ripening within a room with a temperature and humidity controlling system; the procedure of modern processing usually takes 6-8 months [5]. However, with the increase of raw hams weight and the shortening of processing period,spoilage of Jinhua ham frequently happens, especially in location of“three sticks” (sacral vertebra, knee joint and hip joint). Furthermore,the spoilage of the production accounts for annual losses of about 3% of the total economic production.
The spoilage phenomenon is not exterior inspection damage,but it can be smelled with an off-odor in the deep of muscle. The way of grading the Jinhua hams is called “three sticks” method that bamboo sticks are inserted into the sacral vertebra, knee joint and hip joint of hams, and then the aroma adsorbed on the bamboo sticks is evaluated by professional panel [6]. Jinhua ham is commonly divided into 4 grades according to the aroma intensity: “well aroma on three bamboo-sticks” is first grade; “well aroma on two bamboosticks” is the second; “well aroma on one bamboo-stick” is the third,while the fourth grade products are awarded off-odors. Off-odors have been described as rotten note, strawberry-like or yeast-like notes in spoiled hams [7]. Some volatile compounds including acids,alcohols and sulfides showed a positive correlation with off-flavors of spoiled Iberian hams [8,9]. However, little is known about volatile compounds that are associated with the spoilage of “three sticks” in Jinhua ham.
Spoilage of meat products is related to the combination of biological and chemical reactions during the processing or storage [10].Most researchers considered that the microbial populations were mainly responsible for spoilage because of the high nutrient composition, suitable pH and high moisture content in meat products,which allowed the growth and survival of microorganisms [11].The study showed that Enterobacteriaceae could be the main microorganisms which produced metabolites with unpleasant odors involved in ‘bone taint’ spoilage of dry-cured ham [9]. The levels of biogenic amine are associated with consequence of microbial activity in foods such as fermented meat and fish products [12,13]. The microorganisms of Enterobacteriaceae, Clostridium, Staphylococcus and Lactic acid bacteria were associated with the spoilage of Spanish dry-cured ham [14]. However, the key microorganisms responsible for the spoilage of “three sticks” have not yet been identified and discussed in in Jinhua ham.
Therefore, in order to reveal the causes responsible for spoilage in the “three sticks” of Jinhua ham, the “three sticks” of firstgrade hams and fourth-grade hams were sampled randomly from 3 000 Jinhua hams; sensory evaluation, volatile compounds,physicochemical parameters, biogenic amine and microbiological counts were investigated in these hams, and the relationship between microorganisms and spoilage of “three sticks” in Jinhua ham was further discussed in this work.
2. Materials and methods
2.1 Processing of Jinhua ham
Jinhua ham was processed using modern technology at a local factory (Meat Products Corporation of Zhejiang, Yiwu, China). Three thousand fresh hind legs weighing between 12-15 kg were obtained from Landrace. These hams were trimmed into a shape like a bamboo leave. Fresh hams were stacked and salted for 40 days at 4-8 °C and 60%-80% relative humidity, during which time 6% sodium chloride of the total weight of fresh hams was added on four occasions.Sodium nitrite (0.15 g/kg) was mixed in the salt used in the second time. After dry-curing, these hams were soaked and washed with bamboo brushes in warm water (12-15 °C) for 24 h. These hams were fixed on a rack and moved to moderate-temperature fermentation room for fermentation for 2 months, where the ambient temperature and humidity were 15-20 °C and 60%-80%, respectively. Finally,the temperate increased from 20 °C to 35 °C gradually and relative humidity was 60%-75% during the 4-5 months of high-temperature ripening in ripening room.
2.2 Hams grading and samples preparation
The final products of Jinhua ham were assessed by an organoleptic evaluation panel that made up of 10 technicians with extensive experience (more than 7 years) using the traditional “three sticks method”. Generally, the bamboo sticks were put in a cool place to dry for 30 min after soaking in ethanol and then were inserted into the hams at 3 special locations (“three sticks”): sacral vertebra,knee joint and hip joint. The depth of insertion was 5-10 cm. Wellaroma of Jinhua ham referred to a strong meaty odor and without offodor on bamboo stick. The quality of hams was graded according to aroma intensity of bamboo stick in the “three sticks”. Fourth grade hams (n= 20) were randomly selected as spoiled hams, while first grade hams (n= 20) were sampled as normal hams. Microorganisms were aseptically collected by using a sterile metal cork borer from the location of hip joint of normal and spoiled hams, respectively.Meanwhile, the biceps femoris(50 g) of “three sticks” of each ham was immediately taken for sensory evaluation. A series ofbiceps femoris samples (100 g) were taken from the muscle tissue adjacent to hip joint and stored immediately at -40 °C until used for volatile compounds, physicochemical and biogenic amine analysis.
2.3 Sensory evaluation
The sensory descriptors including meaty odor, rancid odor,putrefactive odor, sour odor, sul fide odor and ammonia odor of normal and spoiled hams were performed, according to the study [5]with some modifications. Twenty panelists were trained so that trainees could comprehend testing procedures as well as the evaluation of odors in hams. The value of 0 corresponded to the lowest intensity(not detectable) and the value of 5 to the highest (strongly detectable)for the six odor attributes were defined through panel discussion and references which were provided to the panel for calibration. Prior to analysis, thebiceps femorismuscle samples of normal and spoiled hams were cut into thin slices (3 mm thickness) and then stored at ambient temperature (25 °C) for no more than 15 min before being served. Sensory testing was carried out in a sensory laboratory equipped with individual booths. The assessment was scored using a linear unstructured 1 mm scale anchored at the scales and individual rating for each attribute (meaty odor, rancid odor, putrefactive odor,sour odor, sul fide odor and ammonia odor) did not vary more than ± 0.5 from the average scores.
2.4 Analysis of volatile compounds
The analysis of volatile compounds was conducted according to the study [1]with some modifications. Frozen samples were thawed and minced; 5 g was added into a 20 mL headspace vial (CNW Technologies, Germany) and sealed up with a Te flon/silicone septum in an aluminum cap. 20 μL of 10 ng/μL 2-methyl-3-heptanone was added as an internal standard. Volatile compounds were extracted by solid phase micro extraction (SPME) with a 75 μm carboxyethyl/polydimethylsiloxane (CAR/PDMS) fiber purchased from Supelco Inc. (Bellefonte, PA, USA). Before collecting volatiles, the fiber was preconditioned at 220 °C for 2 h at the GC injection port and extracted in the upper space of the vial for 40 min at 45 °C. After extraction, the fiber was introduced to the injector directly and desorbed for 2 min at 220 °C in the splitless mode. Volatiles analyses were performed using Aglient GC 7890B-MS 7000C system (Agilent Technologies, Little Falls, Del., USA) with a VOCOL capillary column (60 m × 0.32 mm ×1.8 mm) (Supelco, USA). Helium was used as the carrier gas. After desorption, the oven temperature was at 40 °C, holding for 2 min,then ramped to 130 °C at 10 °C/min, and ramped finally to 200 °C at 4 °C/min, maintaining for 20 min. The temperature of ion source and analyzer was at 200 °C and 210 °C. The detector voltage was operated at 0.9 kV. The mass spectrometer scanned in them/z45–600 mass range. Identification was carried out by comparison of mass spectra in the databases of NIST 14, and the final concentration of the volatile substances was calculated based on the concentration of the internal standard substance.
2.5 Determination of moisture, pH, TVB-N and salt contents
The pH meter was calibrated at pH 4.0 and 7.0 with standard buffers (Mallinckrodt Chemicals, Phillipsburgh, USA) stored at room temperature (25 °C). The pH was measured using an electronic pH meter (Mettler Toledo Instruments Co., Ltd., Shanghai, China) in homogenates prepared by homogenizing 2.0 g of muscle samples with 10 volumes of distilled water at room temperature (25 °C).
Salt contents were measured by using a salinometer (Thermo Eutech, CA, USA) in a slurry. Briefly, the slurry was prepared according to the following procedure: 10 g of sample was homogenized in 90 mL of distilled water with an Ultra-Turrax T25(Janke & Kunkel, Staufen, Germany) and then stirred with magnetic stirrer for 20 min at 6 000 r/min.
Moisture content was determined by weight loss on drying of ham samples at 105 °C. Brie fly, the weight loss of 5 g of muscle samples was determined after drying at 105 °C to a constant weight. The TVB-N values were estimated using the semi micro steam distillation.Brie fly, meat sample ((10 ± 0.1) g) was homogenized with distilled water (100 mL, pH 7.0) for 30 min. After filtering, the extract was treated with a magnesia solution (10 g/L) and subjected to steam distillation. The volatile base components were absorbed by a boric acid receiver and determined by titration. The TVB-N values were expressed as mg TVB-N per 100 g ham samples.
2.6 Determination of thiobarbituric acid reactive substances(TBARS)
The TBARS values were evaluated according to previous study by Wang et al. [15]with slight modifications. Briefly, 2 g of ham samples were homogenized in 10 mL of 17.5% trichloroacetic acid(TCA) with a DY89-I high-speed homogenizer (Scientz Co., Ningbo,China) for 20 s at 25 000 r/min while cooled on ice. After filtering the homogenate, 1 mL of 0.02 mol/L thiobarbituric acid (TBA)was added to the filtrate and mixed. The mixture was incubated in a boiling water bath for 40 min. After the solution cooled, the mixture was centrifuged at 2 000 ×gfor 5 min at 4 °C; 1 mL chloroform was added to the supernatant, blended and layered. The absorbance of the upper layer was determined at 532 and 600 nm by a 96-well plate reader M200 (Tecan, Austria). The TBARS values were calculated using the following formula:

The values were expressed as mg of malondialdehyde (MDA) per kg of muscle samples.
2.7 Determination of biogenic amine
Histamine, putrescine, phenylethylamine, cadaverine, tyramine,tryptamine, spermidine, and spermine were purchased from Sigma Company (Shanghai, China). Standard solutions of each biogenic amine (0.5, 1, 2, 5, 20, 50 and 100 μg/mL) were prepared in 0.4 mol/L perchloric acid. Biogenic amine determination was carried out using high performance liquid chromatography (HPLC) according to the method described by Zhang et al. [16]with modification. 5 g of muscle samples was homogenized in 20 mL of 0.4 mol/L perchloric acid and centrifuged at 5 000 ×gfor 10 min at 4 °C. The extraction process was repeated twice under the same conditions. The collected supernatants were combined, and the final volume was adjusted to 50 mL with 0.4 mol/L perchloric acid. Afterward, 1 mL of the solution was mixed with 200 μL of 2 mol/L sodium hydroxide, 300 μL of saturated sodium bicarbonate and 2 mL of 10 mg/mL dansyl chloride (Solarbio Science and Technology Co., Ltd. Beijing, China). The mixture was incubated in a water bath for 30 min at 40 °C without light. Then,100 μL of 25% ammonium hydroxide (Nanjing Chemical Reagent Co., Ltd., Nanjing, China) was added to stop the reaction. Finally,the mixture was adjusted to 5 mL using acetonitrile (Tedia Co., Ltd.,Fair field, OH) and filtered with a 0.22 μm membrane filter.
HPLC was conducted with a ZORBAX XDB-C18column(5 μm, 4.6 mm × 250 mm, Agilent, Santa Clara, CA) at 254 nm. The mobile phase consisted of eluent A (0.1 M ammonium acetate), B(acetonitrile) and C (ultrapure water). The gradient elution program was as follows: 0–6 min, 35%–15% A, 50%–65% B; 6-15 min, 15% A, 65%–68% B; 15-25 min, 15%–0% A, 68%–90% B; 25-25.10 min,0%–35% A, 90%–50% B; 25.10-30 min, 35% A, 50% B. The flow rate, injection volume and column temperature were 1 mL/min, 20 μL and 40 °C, respectively. The content of biogenic amine was calculated according to the standard curves.
2.8 Microbiological analysis
In order to evaluate microbiological enumeration, 25 g ofbiceps femoris from the location of hip joint of the ham was homogenized in 225 mL sterile 0.9% saline solution in a sterile homogeneous bag for 90 s. Appropriate dilutions were made with sterile 0.9% saline solution and 1 mL dilutions were plated onto the culture media under the following conditions: total counts on Plate Count Agar(PCA) for 72 h at 30 °C; Enterobacteriaceae on Violet Red Bile Glucose Agar (VRBGA) for 24 h at 37 °C; lactic acid bacteria on MRS Agar in anaerobic conditions for 72 h at 30 °C; Gram-positive catalase-positive cocci on Mannitol Salt Agar (MSA) after 72 h at 37 °C; sul fite reducing clostridia on Sul fite-Polymyxin-Sulfadiazine(SPS) agar incubated anaerobically for 72 h at 37 °C; intestinalEnterococcuson Slanetz and Bartley agar (S&B) for 24 h at 37 °C;yeasts and moulds on Malt Extract Agar (MEA) for 4 days at 25 °C.For the samples with counts more than 2 lg (CFU/g), the colonies were selected from plates that had counts of 30 to 300 colony counts(2 to 5 colonies per plate) according to their morphology, and were subcultured on the same isolated medium. Each isolate was examined for colony and cell morphology under a microscope.
2.9 Statistical analysis
All statistical analysis was performed using SAS 8.1 software(SAS Institute Inc, USA). The results were expressed as mean values and standard error. The data obtained on the sensory results,volatile compound content, biogenic amine, physicochemical parameters and microbial counts were analyzed statistically usingt-test (P< 0.05).
3. Results and discussion
3.1 The changes of sensory scores between normal and spoiled hams
Fig. 1 shows the results of odor attributes of Jinhua ham from normal and spoiled groups by sensory evaluation. The scores of meaty odor in normal hams were higher than that of spoiled hams (P< 0.05).Conversely, the scores of rancid odor (P< 0.05), putrefactive odor(P< 0.05), sour odor (P< 0.05), sul fide odor (P< 0.05) and ammonia odor (P< 0.05) in spoiled hams were significantly higher than that of normal hams. Lower scores of meaty odor in spoiled hams could be explained by the facts that some compounds with intense rancid and sour odors masked it [8]. The results of sensory evaluation indicated that spoiled and normal hams showed significant difference in odor characteristics.

Fig. 1 Quantitative descriptive sensory evaluation of spoiled and normal hams. Different odor scores of normal and spoiled hams were significant differences (*P < 0.05).
3.2 The changes of volatile compounds between normal and spoiled hams
The contribution of volatile compounds to flavor depends on their threshold values and concentrations, expressed as odor active values(OAVs). As shown in Table 1, the total content of hydrocarbons in normal hams was higher than spoiled hams (P< 0.05), and the OAVs for each component were less than 1 in normal hams, which indicated that they had little contribution to the flavor of Jinhua ham [17].

Table 1Volatile compounds of normal and spoiled hams (μg/kg).

Table 1 (Continued)
For aldehydes, total content of them in normal hams was significantly higher than spoiled hams (P< 0.05). Seven compounds were detected with the high OAVs in normal hams, including 3-methyl butanal (377.2), 2-methyl butanal (42.81), hexanal (191.72),heptanal (21.76), octanal (81.94), nonanal (96.81) and decanal (28.6).These aldehydes were known to be major contributors for distinctive flavor of dry-cured ham because of their rapid formation in lipid oxidation and Strecker reactions [17]. The branched aldehydes, 3-methyl butanal and 2-methyl butanal, were associated with nutty, cheesy and salty odor, and they were produced by proteolysis and Strecker degradation of isoleucine and leucine [18,19].Hexanal was the main substance from the oxidation ofn-6 fatty acids, which possessed a low odor threshold (4.5 μg/kg) and described as putty, fatty and bitter-almond [20]. Heptanal and octanal could contribute to fatty, hamlike and meat-like odor, while nonanal could be related to rancid odor [21].High OAVs of hexanal, heptanal and octanal indicated that they played an important role in formation of meaty odor in Jinhua ham.
With regard to the ketones, total content of normal hams was significantly higher than spoiled hams (P< 0.05). The OAVs of corresponding components in normal hams were less than 1, respectively, while the OAVs of 2,3-butanedione and 2,3-pentanedione were more than 1 in spoiled hams, which could contribute to the caramel and buttery odor [22].
Total content of esters in spoiled hams was significantly higher than normal hams (P< 0.05). Three compounds with the highest OAVs in spoiled hams were methyl isovalerate (157.49), propyl isovalerate (113.21) and ethyl butanoate (59.38). Furthermore, ethyl acetate (1.05), methyl pentanoate (2.45), methyl heptanoate (5.47),methyl butanoate (15.65), methyl hexanoate (9.52), ethyl isovalerate(12.06) and methyl isobutyrate (10.98) were also important odoractive esters of spoiled hams. Ethyl acetate and ethyl isovalerate have been found in naturally spoiled meat products [23], which may be associated with off-odors in spoiled hams.
Total content of alcohols in normal hams was significantly higher than spoiled hams (P< 0.05). 1-octen-3-ol was considered to be responsible for a mushroom note with its low odor threshold (1 μg/kg).It was worth noting that spoiled hams had high OAVs of 3-methyl butanol. One possible metabolic pathway has been demonstrated that alcohols could be oxidized into the corresponding aldehydes and acids [17]. Furthermore, acids could be esterified with alcohols by microbial action [24]. Lower levels of alcohols and higher levels of esters in spoiled hams could be explained by the fact that oxidation of alcohols and esterification were enhanced by the microorganisms activities, compared with normal hams [8,17].
The total content of acids was 1 830.68 μg/kg in spoiled hams,whereas they were not detected in normal hams. The high OAVs(10.5) of butanoic acid in spoiled hams can be explained by the microbial fermentation of free amino acids [8]. Furthermore,butanoic acid usually showed an unpleasant odor [25], which could be closely associated with rancid and sour odors of spoiled hams.Sulphur compounds were detected at 960.01 μg/kg in spoiled hams,which were significantly higher than normal hams (P< 0.05).Methional was only found in normal hams with high OAVs (23.8),which was produced from methionine and related to “cooked potatoes” and meaty odors of normal hams [26]. It was worth nothing that methanethiol was detected in spoiled hams with a high OAVs (324.42), which had some characteristic odors of sewage-like and rotten cabbage [7,27]. Meanwhile, dimethyl disulfide showed higher content (937.30 μg/kg) and OAVs (1 115.83) in spoiled hams than normal hams, which were consistent with previous study [9].This substance with rotten, burnt and spoiled ham odors contributed significantly to the off-odor profile of Serrano ham and Iberian ham [9].Methanethiol and dimethyl disul fide with low odor threshold could be dominant compounds that caused sul fide odor feature of spoiled Jinhua ham.
3.3 The changes of biogenic amine between normal and spoiled hams
The biogenic amine content of spoiled and normal hams are shown in Fig. 2. The total content of biogenic amine in spoiled hams was higher than that of normal hams (P< 0.05). The contents of cadaverine and putrescine in spoiled hams were significantly higher than those in normal hams (P< 0.05), while histamine,2-phenylethylamine and tryptamine were only detected in spoiled hams. The levels of biogenic amine of spoiled hams were higher,compared with the reports of Alfaia et al. [28]. The results implied that excessive hydrolysis of protein and growth of microorganism with decarboxylase activity occurred in spoiled hams [29,30]. In fact, total biogenic amine levels of 1 000 mg/kg in food have been considered dangerous for human health [31]. An upper limit for human consumption has been suggested to be 100 mg/kg of histamine,100-800 mg/kg of tyramine and 300 mg/kg of 2-phenylethylamine in meat products [32]. Thus, the contents of total biogenic amine(206.60 mg/100 g) and histamine (26.81 mg/100 g) in spoiled hams increased the risk of human poisoning. Cadaverine and putrescine were usually accompanied by a disgusting odor and regarded as indicator of spoilage of fish meat [33]. Although they were not identified as toxic substances, they can enhance the effect of histamine and tyramine by interfering with the detoxifying mechanism [34].Spermine was not detected in all experimental groups, which may be due to the utilization of microorganisms as nitrogen source [12,16].Therefore, the putrefactive odor and ammonia odor of spoiled hams could be attributed to cadaverine and putrescine, while histamine could increase the poisoning risk of spoiled hams.
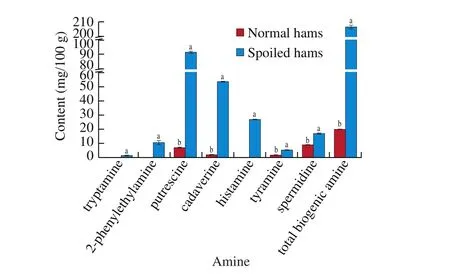
Fig. 2 Biogenic amine levels of normal and spoiled hams (mg/100 g).a-b Different letters in the different groups indicated that there was significant difference (P < 0.05).
3.4 The changes of physicochemical parameters between normal and spoiled hams
The results of physicochemical parameters of two groups are shown in Table 2. The moisture content of spoiled hams was significantly higher compared with normal hams (P< 0.05), while the salt content of normal hams was significantly higher than that of spoiled hams (P< 0.05). Differences in the contents of moisture and salt of dry-cured ham can be attributed to a large number of factors, including raw materials, water activities, salting procedures and ripening process during the production process of dry-cured ham [11,14].However, in the present study, fresh hams were from the same rearing system and were processed under uniform conditions. The differences of moisture and salt contents between normal and spoiled hams could be attributed to the facts that muscle tissues still showed some differences in processing susceptibility even under the same processing conditions, for example salting and dry-ripening [5,8,9].In regard of pH, the value of spoiled hams was significantly higher than normal hams (P< 0.05). The increase in pH seemed mainly to be favored by the increase of microorganism counts in spoiled hams,since some microorganisms have often been reported to be amine producers from amino acid decarboxylation in meat products [35].The concentration of TVB-N in spoiled hams was significantly higher than normal hams (P< 0.05). In fact, in dry-cured meat products,the TVB-N values were typically less than 45 mg/100 g [36], while the value of TVB-N was 116.55 mg/100 g in spoiled hams. The high TVB-N values in spoiled hams could be attributed to the fact that proteins were degraded excessively due to effect of endogenous enzymes and ammonia-producing bacteria, which further formed these compounds including ammonia (NH3), trimethylamine (TMA)and dimethylamine (DMA) [37], and they played a key role in developing the ammonia odor of spoiled hams. The TBARS value of spoiled hams was significantly higher than normal hams (P< 0.05),which implied that more intense lipid oxidation occurred in spoiled hams compared with normal hams. These results indicated that TVB-N and TBARS were important indicators to evaluate the quality characteristics of dry-cured ham between spoiled and normal ham samples [38].

Table 2Physicochemical parameters of spoiled and normal hams.
3.5 Microbial counts of spoiled and normal hams
The microorganism counts of two groups are presented in Table 3.The level of total aerobic bacteria in spoiled hams was significantly higher than normal hams (P< 0.05), which may due to the different environmental conditions of growth, such as high moisture content and suitable pH (Table 2). Gram-positive catalase-positive cocci were the only microbial group isolated from MSA agar. In general,most of them wereStaphylococcus. The counts in spoiled hams were significantly higher than normal hams (P< 0.05). However, it has been reported to be predominant microorganisms during the ripening of Iberian hams [39]. Therefore, it could not be dominant spoilage microorganisms of Jinhua hams.

Table 3Microbiological counts of spoiled and normal hams.
Regarding the Enterobacteriaceae, the counts in spoiled hams were 4.17 lg (CFU/g), whereasthe counts were less than 2 lg (CFU/g)in normal hams.The values were consistent with the results of spoiled Iberian hams [40]. Enterobacteriaceae have been considered as indicators of food safety, which contributed significantly to the spoilage flora on meat products [41]. The high value of putrescine,cadaverine and histamine (Fig. 2) of spoiled hams were related to Enterobacteriaceae, such asC. freundiiandE. aerogenes[13,42],which could be derived from the contamination of the pig carcass during slaughter and fabrication. Furthermore, insufficient salt content in spoiled hams could increase the survival rate of Enterobacteriaceae [14],since some reports have been demonstrated that dry-cured hams could be spoiled if NaCl concentrations did not reach 5% in biceps femoris muscle [14,18].
Lactic acid bacteria counts were more than 2 lg (CFU/g) in the two groups, which were higher than previously reported in Iberian dry-cured ham [40]; compared with normal hams, the populations of spoiled hams were significantly higher (P< 0.05). Some studies suggested that the presence of lactic acid bacteria in dry-cured meat products could produce some acids including lactic acid, butanoic acid and acetic acid [43]. The excessive accumulation of these acids could develop the unpleasant odor of spoiled hams, which was con firmed by the changes of sensory results. The counts ofEnterococcuswere 3.23 lg (CFU/g) in spoiled hams, whereas it was not detected in normal hams. ManyEnterococciwere the producers of tyramine and 2-phenethylamine, but they are not able to produce putrescine and cadaverine [44,45]. The high concentration of methanethiol and dimethyl disul fide in spoiled hams was also related to the activity of Enterobacteriaceae andEnterococci[43]. Furthermore,Enterococcuswas highly resistant to extremes in temperature, pH and salt, which may multiply to high numbers and act as spoilage microorganisms in meat products [46]. Therefore, Enterobacteriaceae andEnterococcimay play important role in developing the spoilage of Jinhua ham.Finally, counts in SPS and MEA culture media were not detected in both the spoiled and normal hams, which was similar to that reported by Alberto et al. [8]. Consequently, microorganisms grown in these media were not considered for characterization and identification of isolates.
4. Conclusion
The off-odors of spoiled Jinhua ham were derived from the putrefactive odor, rancid odor, sul fide odor, sour odor and ammonia odor. Methanethiol, dimethyl disulfide, butanoic acid, cadaverine and putrescine were the main compounds that contributed to the offodors. High levels of TVB-N, TBARS and biogenic amine enhanced the potential hazard to the safety of spoiled hams. High populations of Enterobacteriaceae andEnterococcuscounts contributed to the spoilage of Jinhua ham in location of “three sticks”, and low salt content and high moisture content accelerated the abnormal growth of these microorganisms in spoiled hams.
conflict of interest
The authors declare there is no conflict of interest.
Acknowledgement
This work was supported by National Natural Science Foundation of China (32101975; 32022066; 31871825), National Key Research& Development Program of China (2021YFD2100104), Modern Agricultural Technical Foundation of China (CARS-42-25), Zhejiang Province Natural Science Foundation (LQ22C200017), China Postdoctoral Foundation (2020M681806; 2021T140348), and Science and Technology Programs of Ningbo (202003N4130; 202002N3067).
- 食品科學與人類健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species

