Dynamic changes of peptidome and release of polysaccharide in sea cucumber (Apostichopus japonicus) hydrolysates depending on enzymatic hydrolysis approaches
Yncho Wng, Yn Song, Yogung Chng,b,*, Ynyn Liu, Gungning Chen, Chnghu Xue,b
a College of Food Science and Engineering, Ocean University of China, Qingdao 266003, China
b Laboratory for Marine Drugs and Bioproducts, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
Keywords:
Sea cucumber
Enzymatic hydrolysis
Protein
Peptidome
Polysaccharide
A B S T R A C T
Enzymatic hydrolysis has been widely used to produce bioactive hydrolysates from sea cucumber body wall. Here, inspired by the clarification of Apostichopus japonicus genome, we investigated the enzymatic hydrolysis of sea cucumber body wall by using the omics strategy. Shared proteins, including major yolk proteins, collagens, extracellular matrix glycoproteins and muscle proteins, were released from the body wall by different hydrolysis condition. A portfolio of 216 shared peptides were detected in the peptidome by papain with different hydrolysis time, while 32 shared peptides were detected in the peptidome by differing proteases.Unshared peptides and the relative abundance distribution profiles of shared peptides changed depending on hydrolysis approaches, indicating dynamic changes of peptidome during hydrolysis. Moreover, release of sulfated fucan and fucosylated chondroitin sulfate changed with the hydrolysis condition. The monitoring of dynamic enzymatic hydrolysis process at a molecular scale would contribute to production and quality control of sea cucumber hydrolysates.
1. Introduction
Sea cucumbers are echinoderms in the class Holothuroidea.The sea cucumberApostichopus japonicusis an aquatic food with important commercial value [1,2]. As an example, the total aquaculture production and estimated industrial value of sea cucumbers in China are about 219 kilotons and 7 billion dollars in 2017 [3]. Sea cucumber is comprised of approximately 40%-65% protein and 10% anionic polysaccharide on dry weight basis,mainly including sulfated fucan (also named as fucoidan) and fucosylated chondroitin sulfate [4]. Sea cucumbers belong to proteinrich food material and many protein-derived processing products could be produced from sea cucumbers, for example sea cucumber hydrolysates. Sea cucumber hydrolysates by enzymatic hydrolysis have demonstrated various biological activities, such as antioxidant,angiotensin-converting enzyme inhibition, immunomodulatory, ironbinding, anti-Aβ aggregation and memory-modulation activities [5-9].
Enzymatic hydrolysis has been widely used in the development of traditional and novel sea cucumber derived products, such as sea cucumber hydrolysates. Various protein components in the sea cucumber body wall, belonging to extracellular matrix and muscle proteins, participate in the constitution of sea cucumber body wall.Besides, our previous studies have demonstrated that fucosylated chondroitin sulfate is covalently associated with collagen fibrils [10],and collagen fibrils of sea cucumbers are heterotypic, consisting of various types of collagens [11]. Co nsidering the complex molecule composition of sea cucumber body wall, the investigation of molecule changes during enzymatic hydrolysis would provide a better understanding of the enzymatic hydrolysis process, contributing to the production and quality control of sea cucumber hydrolysates.
The structural properties, such as molecular weight distribution and amino acid sequence, and bioactivities of sea cucumber protein hydrolysates depend on the employed enzymatic hydrolysis approaches [8]. Moreover, previous studies have demonstrated that bioactivities of sea cucumber protein hydrolysates were closely related to their structural properties [8]. As previously reported,hydrolysis time and protease types played critical roles in the production of bioactive protein hydrolysates by enzymatic hydrolysis.Flaxseed protein hydrolysates with different hydrolysis time showed different degree of hydrolysis, exhibiting significantly different angiotensin I-converting enzyme inhibiting activity and hydroxyl radical scavenging activity [12]. Flaxseed protein hydrolysates by alcalase or pancreatin showed significantly higher antioxidant activity in comparison to those by flavorzyme or papain [13]. Enzymatic hydrolysis of sea cucumbers by various types of proteases, especially papain, could liberate proteins from the integrated body wall and have been employed for the preparation of sea cucumber peptides [14].Besides, previous studies have revealed that polysaccharide could release from the sea cucumber body wall during the preparation of protein hydrolysates or peptides by proteases [15]. Considering the various biological activities of sulfated fucan and fucosylated chondroitin sulfate [15-18], these two types of polysaccharide might be regarded as representative ingredients in the sea cucumber hydrolysates. Nevertheless, how the peptides and polysaccharide were released from the sea cucumber body wall during the enzymatic hydrolysis process remains unclear.
Protein hydrolysates represent a complex mixture of peptides,which makes the identification and classification of peptide sequences from respective parent proteins by conventional techniques a challenge. Proteomics technique, as well as peptidomics technique encompass the qualitative and quantitative characterization of entire protein and peptide components in an organism. Recently, the genome and proteome of sea cucumber (Apostichopus japonicus) have been published in the NCBI database [19]. Having these sequences available would enable us to investigate sea cucumber hydrolysates by using an omics strategy in the post-genome era. Here, the aim of this study was to investigate the molecular changes in sea cucumber hydrolysates from the body wall depending on the enzymatic hydrolysis approaches by using a proteome-based approach, including 1) release of proteins and changes of peptidome in the sea cucumber hydrolysates during the enzymatic hydrolysis process; 2) release of polysaccharide in the sea cucumber hydrolysates during the enzymatic hydrolysis process.
2. Materials and methods
2.1 Materials
Fresh sea cucumber (A. japonicus) was purchased from a local aquatic market in Qingdao, China. Papain, alcalase, neutrase and flavorzyme were purchased from Pangbo Biological Engineering Co.,Ltd. (Nanning, China).
2.2 Enzymatic hydrolysis of sea cucumber body wall
Sea cucumbers were immediately dissected, gutted and cleaned with distilled water at 4 °C. The attached viscera and longitudinal muscle layers on the inside were pulled off. Both the pigmented outer dermis and circular muscles were included in the body wall. The sea cucumber body wall was cut into pieces and homogenized by using a blender. Sea cucumber body wall (155.73 g) was suspended in distilled water (1/5,m/m), and the mixture was adjusted to appropriate pH value with the addition of 2 mol/L NaOH or 2 mol/L HCl (papain,pH 7.0; alcalase, pH 8.5; neutrase, pH 7.0; flavorzyme, pH 7.0).Subsequently, enzyme (papain, alcalase, neutrase or flavorzyme, 0.23 g)was added into each suspension respectively and the mixture was incubated at the appropriate temperature (papain, 60 °C; alcalase,45 °C; neutrase, 55 °C; flavorzyme, 45 °C) with continuous stirring.Samples were collected from the mixture at different intervals(5, 30, 60, 120, 480 min), deactivated in the boiling water bath for 10 min, rapidly cooled with cold water, and subsequently stored at-80 °C for further analysis. Hydrolysates by papain with hydrolysis time of 5, 30, 60, 120 and 480 min were coded as papain5, papain30,papain60, papain120 and papain480, respectively. Hydrolysates by alcalase, neutrase and flavorzyme with hydrolysis time of 480 min were coded as alcalase480, neutrase480 and flavorzyme480,respectively.
2.3 Chemical composition analysis
The soluble protein contents of samples were measured by the Folin-phenol reagent method [20]with bovine serum albumin as a standard. The soluble anionic polysaccharide contents of samples were measured by the dimethylmethylene blue colorimetry method at 560 nm [21]. The standard curve was constructed with fucoidan as a standard. The monosaccharide composition was determined by a highperformance liquid chromatography (HPLC) method with 1-phenyl-3-methyl-5-pyrazolone derivatization [22].
2.4 Size exclusion chromatography
The soluble protein and polysaccharide components in the sea cucumber hydrolysates were analyzed by size exclusion chromatography using an AKTAprime plus system (GE, United States) equipped on a Hiprep 16/60 Sephacryl S-500 column(16 mm × 600 mm, GE). Phosphate buffer solution (50 mmol/L NaH2PO4-Na2HPO4, 0.2 mol/L NaCl, pH 7.0) was used as eluent at a flow rate of 0.5 mL/min with an injection volume of 4 mL.The sample solution was filtered through 0.22 μm microfiltration membrane and loaded at a flow rate of 0.3 mL/min. The elution of protein was monitored at 280 nm. The elution was collected at an 8-min interval and the collected elution fraction was used for anionic polysaccharide content determination. The polysaccharide components were identified by using monosaccharide composition analysis [22]. The column was calibrated with a series of dextran standards (molecular weight 1 400, 670, 150, 12 kDa).
2.5 Peptide sequence analysis by liquid chromatographytandem mass spectrometry (LC-MS/MS)
Before peptide sequence analysis by LC-MS/MS, the sample was deproteinized according to the method of Khaldi [23]. Briefly, the sample solution was mixed with equal volume of 20% trichloroacetic acid solution, left at 4 °C for 30 min and centrifugated at 15 000 ×gfor 20 min. Subsequently, the supernatant was desalted by using an Empore SPE Cartridges C18column. The C18column was activated with methanol and equilibrate with acetonitrile (0.1% formic acid).Subsequently, the sample was loaded onto the column, desalted with 5% acetonitrile (0.1% formic acid), eluted with acetonitrile, and then subjected to vacuum drying.
LC-MS/MS analysis was performed by using an Ultimate 3000 RSLCnano system (Thermo, Waltham, MA, USA) coupled to a Q Exactive mass spectrometer (Thermo, Waltham, MA, USA). The system was equipped with an Acclaim PepMap C18column (300 μm ×5 mm, 5 μm, Thermo) and an Acclaim PepMap C18analytical column(75 μm × 150 mm, 3 μm, Thermo). Dried sample was dissolved in buffer (2% acetonitrile, 98% H2O, 0.1% formic acid) and separated at a flow rate of 300 nL/min. A binary gradient elution system,comprising buffer A (100% H2O, 0.1% formic acid) and buffer B(80% acetonitrile, 20% H2O, 0.1% formic acid), was applied as follows: from 0–5 min, 5% B; 5-45 min, 5%–50% B; 45-50 min,50%–90% B; 50-55 min, 90% B; 55-65 min, 90%–5% B. Data were acquired with the following MS conditions: ESI source, positive mode; acquisition mode, data dependent top 20 mode; MS scan range,m/z350-1 800; MS scan resolution, 70 000; MS/MS scan resolution,17 500; normalized collision energy, 27%.
Raw data files were searched using Proteome Discoverer 2.1 againstA. japonicusproteome (ASM275485v1) database downloaded from NCBI [19]. The search parameters were set as follows: enzyme specificity was set to unspecific; potential dynamic modifications were oxidation (M) and oxidation (P); precursor mass tolerance was set to 10-6;fragment mass tolerance was set to 0.02 Da. Peptides identification results were filtered with a false discovery rate (FDR) ≤ 1%.The peak areas of precursor ions were used for peptide abundance determination. Proteins containing at least one peptide were used for protein identification and protein identification results were filtered with a FDR ≤ 1%. Heatmap graphs were performed using R software(version 3.6.3, R Foundation for Statistical Computing, Vienna,Austria). The peptide abundance was transformed into the logarithmic format (log2Abundance) and normalized among the same sample to display the relative peptide abundance distribution profile. Euclidean distance was used for hierarchical clustering analysis.
2.6 Biological activity prediction
Potential anti-inflammatory and antihypertensive activities of peptides were predicted by using AntiInflam (http://metagenomics.iiserb.ac.in/antiinflam/pred.php) and mAHTPred [24](http://thegleelab.org/mAHTPred) software respectively. Support vector machine scores were set to 0.5 for anti-inflammatory and antihypertensive peptide prediction.
2.7 Statistical analysis
Data were analyzed from three independent experiments and expressed as mean ± SD. Statistical analysis was performed using one-way analysis of variance (ANOVA). Tukey’s post hoc test was made for multiple comparisons if one-way ANOVA test was found to be statistically significant. Statistical significance was set atP< 0.05.All statistical analysis was performed using SPSS 25.0.
3. Results and discussion
3.1 Molecular changes in sea cucumber hydrolysates during enzymatic hydrolysis by papain
3.1.1 Release of proteins and polysaccharide by papain with different hydrolysis time
Enzymatic hydrolysis has developed into one of the most widely employed bioprocess techniques to recover proteins and polysaccharide from food materials or food byproducts, which could be regarded as modified water extraction process with the assistance of enzymes.According to our previous studies, 472 structural proteins have been detected in the sea cucumber body wall, including collagens,proteoglycans, extracellular matrix glycoproteins, muscle proteins, and proteases [25]. Our preliminary experiments demonstrated that major yolk proteins and sulfated fucan were detected to be the major soluble protein and polysaccharide components in the water extraction process without the assistance of enzymes.
Papain has been widely used for the preparation of hydrolysates from sea cucumber body wall by using enzymatic hydrolysis approach [26]. For papain5, the soluble protein content((2.22 ± 0.01) mg/mL) and presence of an obvious elution peak at OD280nmindicated the existence of soluble protein components in the sea cucumber body wall (Fig. 1A), which was in good agreement with previously detected soluble protein components in the water extract of sea cucumber body wall. The soluble protein concentration in sea cucumber hydrolysates increased with the increasing of hydrolysis time, i. e., (2.22 ± 0.11) mg/mL for papain5, (4.91 ± 0.07) mg/mL for papain30, (5.32 ± 0.10) mg/mL for papain60, (5.61 ± 0.09) mg/mL for papain120, and (5.78 ± 0.10) mg/mL for papain480 (Fig. 1A). This was consistent with previous reported studies, which demonstrated that the release of TCA-soluble proteins during hydrolysis of hemp protein isolate by various proteases increased with the increasing of hydrolysis time [27]. Accordingly, protein components were released from the sea cucumber body wall along with the increasing of enzymatic hydrolysis time.
Two distinct peaks were observed in the distribution profile of soluble polysaccharide (Fig. 1B), suggesting the presence of two types of polysaccharide. Sample from the first elution peak at the volume of 44 mL was mainly comprised of fucose, corresponding to the sulfated fucan (Fig. S1). Sample from the second peak at the volume of 84 mL consisted of mannose, glucosamine, galactosamine,glucuronic acid, glucose and fucose, corresponding to the fucosylated chondroitin sulfate (Fig. S1). The concentrations of polysaccharide in papain5, papain30, papain60, papain120 and papain480 were(1.53 ± 0.04), (2.49 ± 0.07), (3.03 ± 0.01), (3.12 ± 0.08) and (3.15 ±0.08) mg/mL, respectively, suggesting that soluble polysaccharide contents in sea cucumber hydrolysates significantly increased with the increasing of hydrolysis time during the first 60 min (P< 0.05).In comparison to other types of marine protein hydrolysates, such as fish protein hydrolysates and krill protein hydrolysates, sea cucumber protein hydrolysates consisted of a considerable amount of anionic polysaccharide, i.e. sulfated fucan and fucosylated chondroitin sulfate,suggesting that these two types of polysaccharide might be considered as the characteristic ingredients of sea cucumber hydrolysates by protease enzymatic hydrolysis.

Fig. 1 The solubilization of (A) proteins and (B) polysaccharide from sea cucumber body wall during enzymatic hydrolysis by papain with the increasing of hydrolysis time. Calibration standards were marked in the figure from left to right as follows: 1 400, 670, 150, and 12 kDa. The total soluble protein contents (mg/mL) in the solution were labeled in the legend.
Various types of proteins were detected in sea cucumber hydrolysates by papain, and the molecular species of detected proteins in the hydrolysates varied with the increasing of hydrolysis time(Fig. 2A). For papain30, a mass of peptide sequences, corresponding to 219 proteins, were detected in the hydrolysates. With the increasing of hydrolysis time, peptide sequences belonging to more proteins were detected in the hydrolysates in comparison with those in papain30. A set of 67 shared proteins, mainly including major yolk proteins, muscle proteins, collagens and other extracellular matrix proteins, were detected in the hydrolysates by papain with differing hydrolysis time (Fig. 2A and Supplemental Data S1). The number of detected peptide sequences from individual shared proteins in the hydrolysates changed with the increasing of reaction time, which resulted from the degradation of proteins or further degradation of existed peptide fractions (Fig. 2B). Regardless of the discrepant signal responses of peptides, the number of detected peptide sequences mainly depends on the contents of their parent proteins and the degradation availability of parent proteins by respective proteases.Collagens account for about 70% of the total protein mass of the sea cucumber body wall [28], and several shared collagens (PIK60693.1,PIK60696.1, PIK44932.1, PIK60691.1, PIK60692.1, PIK62545.1,and PIK62546.1) were identified in the protein hydrolysates by papain with different enzymatic hydrolysis time (Fig. 2A). Four types of shared muscle proteins, including myosins (PIK40516.1),tropomyosins (PIK44187.1), actins (PIK49311.1) and actin-binding proteins (PIK33909.1, PIK49804.1, and PIK47428.1), were detected in the protein hydrolysates, which were consistent with the presence of muscle layers in the sea cucumber body wall [25]. Interestingly,over 150 peptides from major yolk proteins (PIK45785.1,PIK45784.1, PIK45783.1 and PIK46227.1) were detected in sea cucumber hydrolysates by papain. Our preliminary experiments confirmed that sea cucumber body wall consisted of some watersoluble protein components, and major yolk proteins were present in the soluble protein component with relative high abundance through LC-MS analysis. Major yolk protein, a substantial constituent of the coelomic fluid irrespective of sex and reproductive season, has been determined as the most abundant protein in the coelomic fluid of sea cucumber (A. japonicus) [29]. Transcripts of major yolk proteins have been detected in various tissues and organs of sea cucumbers, such as body wall, intestine, coelomocytes, testis, and ovary [29]. Amino acid sequences of sea cucumber major yolk protein shared a 29% identity with those of sea urchin major yolk protein, which was a member of the transferrin superfamily of iron binding proteins [30]. Previous studies demonstrated that peptides from ovotransferrin showed many biological activities, such as anti-inflammatory, antimicrobial and antioxidant activities [31-33]. Presumably, major yolk protein derived peptides of the sea cucumber body wall might possess some potential biological activities. According to the above analysis, changes of released proteins were involved in the enzymatic hydrolysis process,and the release of individual protein molecules was related with the enzymatic hydrolysis.

Fig. 2 Dynamic changes of proteins and peptidome for sea cucumber hydrolysates by papain with the increasing of hydrolysis time. (A) Venn diagrams of identified protein components; (B) The number of identified peptide sequences in representative proteins; (C) Venn diagrams of total identified peptides; (D) The number of shared peptides in representative proteins.
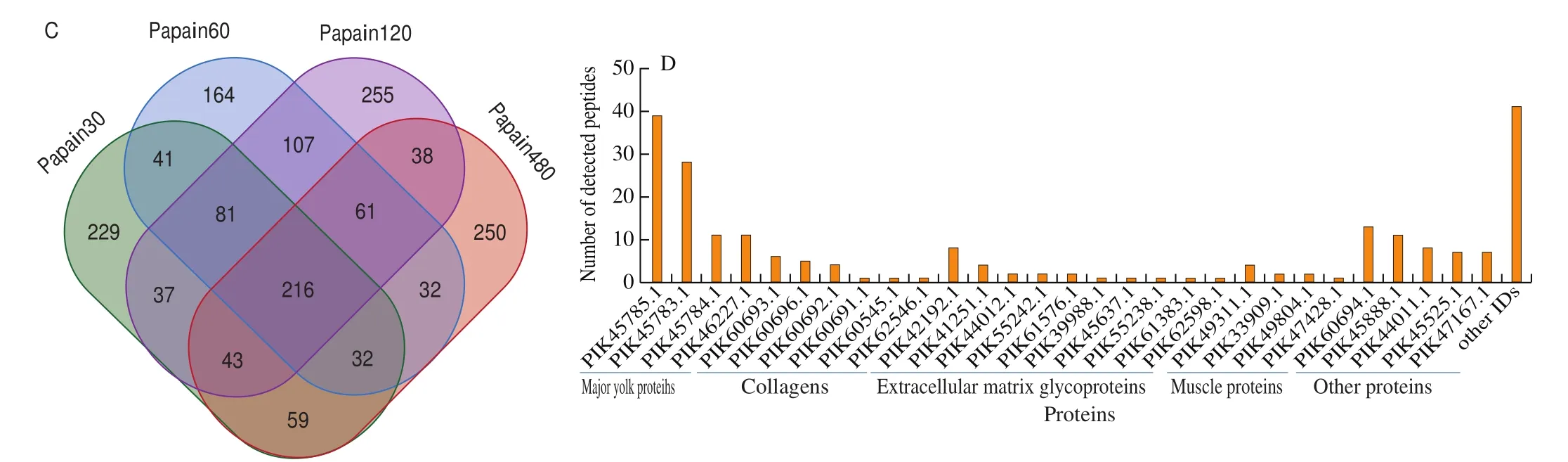
Fig. 2 (Continued)
3.1.2 Changes of peptidome by papain with different hydrolysis time
A large number of peptides were detected in sea cucumber hydrolysates by papain, i.e., 738 peptides for papain30, 734 peptides for papain60, 838 peptides for papain120 and 731 peptides for papain480 (Fig. 2C). A set of 216 shared peptide fractions, mainly belonging to major yolk proteins, collagens, extracellular matrix glycoproteins and muscle proteins, were found in the hydrolysates by papain with differing hydrolysis time (Fig. 2D and Supplemental Data S2). Nevertheless, the relative abundance of 216 shared peptides in the hydrolysates by papain with different hydrolysis time showed different distribution profiles, indicating the dynamic changes of peptides depending on the hydrolysis time (Fig. 3A).In which, some shared peptides showed relative stable distribution profiles, which might be considered as potential characteristic peptides of sea cucumber hydrolysates. Among the shared peptides,78 of 216 peptide sequences were released from major yolk proteins(PIK45783.1, PIK45784.1, PIK45785.1), and showed relative high abundances among the shared peptide fractions. Papain30 shared more identical peptides with papain60 and papain120 than papain480,suggesting more novel peptides were generated with the increasing of hydrolysis time (Fig. 2C). Peptides might undergo further degradation and generate novel short peptide fractions with the increasing of hydrolysis time. As an example, the generation of HELNWGIPGL(papain30/60/120/480) and HELNWGIPG (papain60/480) might result from the degradation of HELNWGIPGLSF (papain30/60/120)or other longer sequences.

Fig. 3 Dynamic changes of peptidome for sea cucumber hydrolysates by papain with the increasing of hydrolysis time. (A) Relative abundance of peptides in sea cucumber hydrolysates; (B-E) The terminal amino acid frequency of identified peptides (papain30, papain60, papain120, papain480).
As shown in Figs. 3B-3E, for sea cucumber hydrolysates by papain with differing hydrolysis time, terminal amino acids and their adjacent amino acids of peptide sequences showed consistent occurrence frequency. Most of peptide sequences in sea cucumber hydrolysates by papain contained relative conservative C-terminal amino acids, especially the second position at C-terminal with preference to hydrophobic amino acids. Proteases play critical roles in the degradation of proteins and the specificity of a protease determines the position at which the protease would catalyze peptide bond hydrolysis [34]. In this current study, the production of sea cucumber hydrolysates resulted from the synergetic hydrolysis of autolytic enzymes and exogenous enzymes. A few types of autolysis enzymes,mainly including metalloproteinases [35], cysteine proteases [36,37]and aspartate proteases [38], have been isolated from sea cucumber body wall, which could hydrolyze collagen and non-collagen proteins.Papain prefers amino acid sequences bearing a large hydrophobic side chain at the second position atN-terminal, and the terminal amino acid occurrence frequency of peptides in sea cucumber hydrolysates was in good agreement with the hydrolysis behavior of papain. Accordingly, individual peptide sequences in sea cucumber hydrolysates by papain dynamically changed with the increasing of hydrolysis time, however, these peptide sequences shared consistent terminal amino acid distribution frequency profiles.
Various peptide sequences in the hydrolysates by papain showed similar structure motifs to previously reported antihypertensive peptides, suggesting potentials in reducing blood pressure (Fig. 4A and Supplemental Data S3). This was consistent with previously reported antihypertensive activities of sea cucumber peptides [6].Besides, approximately 100 putative anti-inflammatory peptides were detected in the hydrolysates by papain, containing similar sequence motifs to reported anti-inflammatory molecules (Fig. 4A and Supplemental Data S4). Hypertensive has been considered as one of the most important risk factors in the development of cardiovascular diseases. Moreover, the role of inflammation in the pathophysiology of cardiovascular disease risk factors, such as hypertensive, is well recognized [39]. The presence of various predicted antihypertensive peptides and anti-inflammatory peptides confirmed the potential of sea cucumber hydrolysates against major risk factors of cardiovascular diseases. Protein hydrolysates by papain with differing hydrolysis time shared 32 identical predicted antihypertensive peptides and 28 identical predicted anti-inflammatory sequences,which might result in differing respective activities (Figs. 4B, C).The differing bioactivities of peptides might attribute to their discrepant amino acid sequences and chain length. Previous studies have reported that enzymatic hydrolysis with differing hydrolysis time might result in protein hydrolysates with discrepant biological activities [40,41]. Interestingly, only 22 shared sequences were found in the predicted antihypertensive dataset and anti-inflammatory dataset (Fig. 4D), suggesting that the protein hydrolysates might prevent cardiovascular diseases through the cooperation of peptides with different biological activities. The described association between sea cucumber hydrolysates and cardiovascular diseases should be interpreted with caution and need further experimental verification.However, our results demonstrated that the potential bioactivities of hydrolysates might change with the dynamic changes of peptidome in sea cucumber hydrolysates depending on different hydrolysis time.

Fig. 4 Predicted bioactive peptides of sea cucumber hydrolysates by papain with the increasing of hydrolysis time. (A) The number of predicted antihypertensive and anti-inflammatory peptides; (B) Venn diagrams of predicted anti-inflammatory peptides; (C) Venn diagrams of predicted antihypertensive peptides; (D) Venn diagrams of total predicted antihypertensive and anti-inflammatory peptides.
3.2 Molecular changes in sea cucumber hydrolysates during enzymatic hydrolysis by differing types of proteases
3.2.1 Release of proteins and polysaccharide by different proteases
Various types of proteases have been employed to recover protein and protein-derived peptides from marine animals, especially alcalase,neutrase, papain and flavorzyme. Changes of protein distribution profiles resulted from the degradation of proteins in the body wall by different types of proteases with the increasing of hydrolysis time(Fig. 5). For sea cucumber hydrolysates by all 4 types of proteases,contents of soluble proteins in the samples significantly increased with the increasing of hydrolysis time (P< 0.05, Fig. 5). Sea cucumber hydrolysates by alcalase and neutrase showed similar protein distribution profile along with the elution volume (Figs. 5A and C).The release of peptides in sea cucumber hydrolysates was closely related to the degradation of proteins in the body wall, which was greatly depended on the synergistic effect of endogenous and exogenous proteases. The discrepancy protein distribution profiles suggested that exogenous proteases played critical roles in the release of proteins under the experimental enzymatic hydrolysis condition.

Fig. 5 The solubilization of proteins and polysaccharides from sea cucumber body wall during enzymatic hydrolysis by different proteases with the increasing of hydrolysis time. Calibration standards were marked in the figure from left to right as follows: 1 400, 670, 150, and 12 kDa. The total soluble protein contents (mg/mL) in the solution were labeled in the legend.

Fig. 5 (Continued)
For all the samples, the presence of two distinct peaks in the polysaccharide distribution profiles, corresponding to sulfated fucan and fucosylated chondroitin sulfate, was observed (Fig. 5). The peak values of elution peaks for fucosylated chondroitin sulfate changed with the increasing of hydrolysis time (Fig. 5). Our previous studies have confirmed that fucosylated chondroitin sulfate is covalently associated with collagen fibrils in sea cucumberA. japonicusbody wall [10]. The release of fucosylated chondroitin sulfate from the sea cucumber body wall might result from the degradation of protein by proteases, leading to the changes of polysaccharide distribution profiles with the increasing of hydrolysis time. The obvious elution peak of sulfated fucan for hydrolysates with the hydrolysis time of 5 min suggested that sulfated fucan might be present as fillings in the sea cucumber body wall (Fig. 5). This was consistent with our preliminary studies, which demonstrated that sulfated fucan was present in the water extract of sea cucumber body wall as the major polysaccharide components. The concentrations of polysaccharides in papain480, alcalase480, neutrase480 and flavorzyme480 were(3.15 ± 0.08), (2.88 ± 0.04), (3.01 ± 0.01) and (2.75 ± 0.02) mg/mL,respectively. Discrepancy in the polysaccharide concentrations mainly resulted from the release of sulfated fucan and fucosylated chondroitin sulfate during the enzymatic hydrolysis process.
Difference in the detected protein molecules among hydrolysates by differing types of proteases was observed (Fig. 6A). A set of 240 proteins were detected in papain480, while 116, 142 and 244 proteins were detected in alcalase480, neutrase480, and flavorzyme480 respectively. A set of 27 shared proteins were detected in the hydrolysates by these 4 types of proteases, mainly including major yolk proteins, collagens, and extracellular matrix proteins (Fig. 6A and Supplemental Data S5). For these shared proteins, a very large proportion of detected peptides were derived from collagens, confirming the occurrence of collagen degradation during the enzymatic hydrolysis. Previous studies have done a mass of work on collagen or collagen peptides from sea cucumbers, which have provided important scientific evidence for application of sea cucumber hydrolysates. Moreover, discrepancy in the number of detected peptides from individual shared proteins was observed among samples by differing types of proteases due to their different hydrolysis behavior (Fig. 6B).
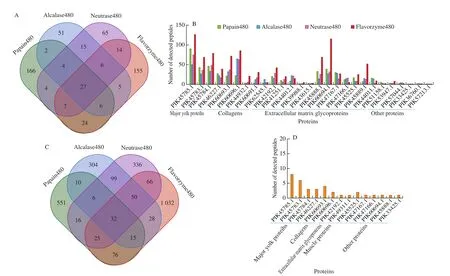
Fig. 6 Dynamic changes of proteins and peptidome for sea cucumber hydrolysates by different proteases. (A) Venn diagrams of identified protein components;(B) The number of identified peptide sequences in representative proteins; (C) Venn diagrams of identified peptides from collagens; (D) The number of shared peptides in representative proteins; (E) Relative abundance of peptides in sea cucumber hydrolysates; (F-I) The terminal amino acid frequency of identified peptides (alcalase480, neutrase480, papain480, flavorzyme480).

Fig. 6 (Continued)
3.2.2 Changes of peptidome by different proteases
A set of 731 peptides were detected in papain480, while 544,630 and 1324 peptides were detected in the hydrolysates by alcalase,neutrase, and flavorzyme with the hydrolysis time of 480 min respectively (Fig. 6C). For hydrolysates by the 4 types of proteases,32 shared peptide sequences were detected and a large number of non-shared peptide sequences were detected in respective samples(Fig. 6C, Supplemental Data S6). The relative abundance of shared peptides in the hydrolysates by different types of proteases showed different distribution profiles, confirming the dynamic changes of peptides during enzymatic hydrolysis process and the critical role of proteases in controlling the production of peptide sequences (Fig. 6E).Peptides, such as LTPTYPEFLDTMK, TPTYPEFLDTMK,GLGDILNDIPDEIR and SVHDEQYEWIK, could be detected in the hydrolysates by 4 different proteases with relative high abundances,suggesting that these sequences might be potential characteristic sequences for the quality control of sea cucumber hydrolysates.Alcalase is a serine endopeptidase with broad specificity and a preference to cleave after hydrophobic amino acids. Neutrase belongs to one type of endo-metalloproteases, which generally shows no hydrolysis specificity. In this current study, enzymatic hydrolysis by alcalase or neutrase resulted in a mass of peptide sequences with less conservative terminal amino acids comparing to that by papain(Figs. 6F and G), which was consistent with the broad specificity of alcalase and neutrase.
Various types of proteases have been employed to hydrolyze sea cucumber proteins and the generated hydrolysates demonstrated differing biological activities [5,8]. Sea cucumber protein hydrolysates by flavorzyme contained more predicted antihypertensive peptides in comparison to those by other three employed proteases (Fig. 7 and Supplemental Data S3). Moreover, more predicted anti-inflammatory sequences were detected in the protein hydrolysates by papain in comparison to the other three samples (Fig. 7 and Supplemental Data S4). Discrepancy in preferred predicted biological activities of protein hydrolysates by differing proteases demonstrated the critical role of proteases in the production of protein hydrolysates with desired bioactivities. Considering the occurrence of inflammation in the development of cardiovascular diseases, the compound product of protein hydrolysates by differing proteases might lead to better prevention against cardiovascular diseases.
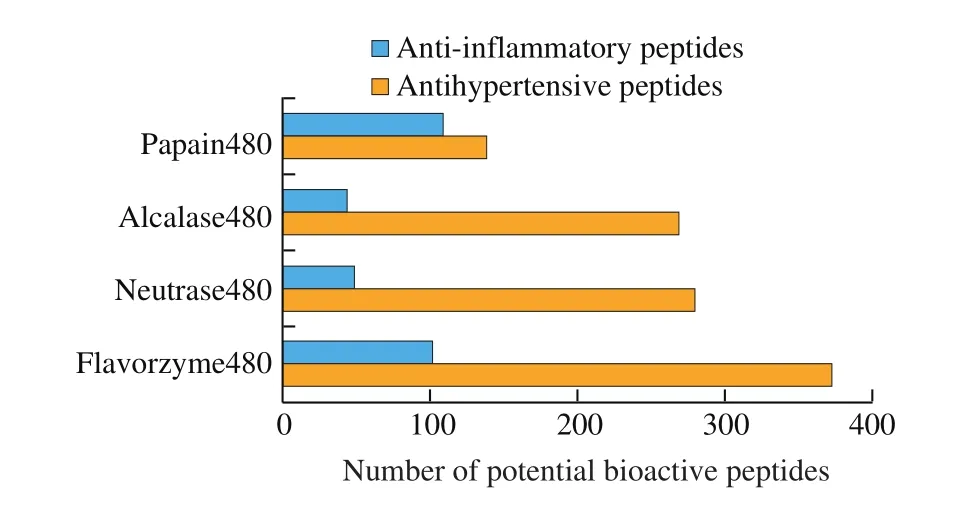
Fig. 7 The number of predicted bioactive peptides of sea cucumber hydrolysates by different proteases.
Enzymatic hydrolysis has been widely used to produce bioactive hydrolysates from sea cucumber body wall. Most of the previous studies on enzymatic hydrolysis of sea cucumbers focused on the characterization of protein hydrolysates, such as degree of hydrolysis and average molecular weight, and their biological activities. Here, we investigated the enzymatic hydrolysis of sea cucumber body wall at a molecular scale and demonstrated the dynamic changes of peptidome and release of polysaccharide in sea cucumber hydrolysates during enzymatic hydrolysis, and further confirmed the critical role of proteases in the production of desired bioactive peptides. Moreover,the release of characterized polysaccharides, i.e. sulfated fucan and fucosylated chondroitin sulfate, which could be also considered as characteristic ingredients in sea cucumber hydrolysates, were determined depending on enzymatic hydrolysis approaches. Protease enzymatic hydrolysis of sea cucumber body wall manifested as a dynamic process with the increasing of hydrolysis time, accompanied by peptidome variation and polysaccharide release.
4. Conclusion
This study demonstrated the dynamic changes of peptidome and release of anionic polysaccharide depending on enzymatic hydrolysis approaches, and confirmed the presence of shared peptides and two types of polysaccharide, i.e. sulfated fucan and fucosylated chondroitin sulfate, in the sea cucumber hydrolysates during enzymatic hydrolysis, which might be considered as potential characteristic ingredients for quality control. Various protein molecules, mainly including major yolk proteins, collagens,extracellular matrix glycoproteins and muscle proteins, were released with the development of enzymatic hydrolysis by different types of proteases, and the release behavior depended on the employed enzymatic hydrolysis time. Peptidome by papain with different hydrolysis time showed consistent terminal amino acid distribution frequency, while peptidome by different proteases displayed discrepancy terminal amino acid distribution frequency. A portfolio of 216 shared peptides were detected in the peptidome by papain with different hydrolysis time, while 32 shared peptides were detected in the peptidome by differing proteases. Moreover, the relative abundance distribution profiles of shared peptides changed depending on enzymatic hydrolysis approaches, indicating the dynamic changes of peptidome during enzymatic hydrolysis. The present study investigated the enzymatic hydrolysis of sea cucumbers at a molecular scale, contributing to a better understanding of the production and quality of sea cucumber protein-derived products.
Declaration of conflicting interest
The authors declare no competing financial interest.
Acknowledgement
This work was supported by the Fok Ying-Tong Education Foundation (171024), Fundamental Research Funds for the Central Universities (201941005) and Fundamental Research Funds for the Central Universities (862001013136).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.04.025.
- 食品科學與人類健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species

