Sulforaphane enhances Nrf2-mediated antioxidant responses of skeletal muscle induced by exhaustive exercise in HIIT mice
Yngwenjie Wng, Yng Xing, Ruiqi Wng, Xingning Li,Jinxiong Wng, Siwng Yu, Ying Zhng,*
a School of Sport Science, Beijing Sport University, Beijing 100084, China
b College of Physical Education, Yan’an University, Shaanxi 716000, China
c Faculty of Health, Engineering, and Sciences, University of Southern Queensland, Toowoomba 4350, Australia
d State Key Laboratory of Natural and Biomimetic Drugs, Department of Molecular and Cellular Pharmacology,Peking University School of Pharmaceutical Sciences, Beijing 100191, China
Keywords:
Sulforaphane
Nrf2 (nuclear factor erythroid-derived 2-like 2)
Skeletal muscle
Antioxidant
HIIT (high-intensity interval training)
Mice
A B S T R A C T
Nuclear factor erythroid-derived 2-like 2 (Nrf2) is the master regulator of antioxidant defenses. High-intensity interval training (HIIT) has been proposed as a time-efficient training program and has become a substantial component of modern training program. In the present study, we evaluated the effects of sulforaphane (SFN),a dietary isothiocyanate derived from cruciferous vegetables and a potent Nrf2 activator, on Nrf2-mediated antioxidant defense responses of skeletal muscle induced by exhaustive exercise in HIIT mice.Male C57BL/6J mice were randomly allocated into control group, HIIT group, and HIIT pretreated with SFN(HIIT+SFN) group. On the third day after completion of a 6-weeks HIIT protocol, an exhaustive treadmill test was conducted in all mice. Mice were intraperitoneally injected with SFN (HIIT+SFN group) or PBS(HIIT and control mice) 4 times in 3 days prior to the exhaustive treadmill test. The results indicated that the 6-weeks HIIT protocol did not increase the antioxidative capacity of skeletal muscle during exhaustive exercise. Importantly, SFN treatment improved antioxidative capacity of skeletal muscle in response to the acute exhaustive exercise by increasing mRNA and nucleoprotein expression of Nrf2 and these genes involved in antioxidant generation and decreasing blood creatine kinase (CK) and 4-hydroxy-2-nonenal (4-HNE)-modified protein levels in the HIIT mice.
1. Introduction
Nuclear factor erythroid-derived 2-like 2 (Nrf2) is a reactive oxygen species (ROS) sensitive transcription factor. Exposure of cells to oxidative stress causes Nrf2 to translocate from the cytoplasm to the nucleus, where it binds to the antioxidant response element,and then to initiate defense that protect the cells against cytotoxic and oxidative damage [1,2]. It has been reviewed that Nrf2 is the master regulator of antioxidant defenses and regulates more than 200 cytoprotective genes in response to oxidative stress [3-5]. Prolonged strenuous exercise increases the production of ROS [6,7]and causes structural damage and contractile dysfunction in skeletal muscle [8].It is logical to deduce that the modulation of Nrf2-related antioxidant signaling may preserve redox and functional homeostasis in the heavily exercised muscles [9,10].
Sulforaphane (SFN), a natural dietary isothiocyanate in cruciferous vegetables, such as broccoli and cabbage, is a specific activator of Nrf2 [11,12]. It has been reported that SFN supplementation increased the running distance of C57BL/6J mice,compared with that of the control mice. Enhanced running capacity was accompanied by the up-regulation of Nrf2 signaling and its downstream genes [13]. Moreover, SFN treatment protects skeletal muscle against the damage induced by exhaustive exercise in rats [14].Our team has also reported that SFN increased monocarboxylate transporter 1 (MCT1) expression in mouse skeletal muscle after acute hypoxic exercise. This result suggests that Nrf2 activation by SFN is a promising strategy to enhance exercise endurance under hypoxia for normal mice [15]. Current studies about the relationship between SFN treatment and exercise capacity primarily focus on untrained mice or rats; however, the effects of SFN on Nrf2 activation in skeletal muscle of exercise trained animals induced by acute exhaustive exercise remain unexplored.
High-intensity interval training (HIIT) program consists of brief intervals of vigorous exercise interspersed with periods of low intensity exercise or rest [16]and has become a substantial component of modern training program, especially in the intermittent sports, such as team or racket sports [17]. Previous studies have shown positive effects of this training program on endurance performance [18-20].There is a growing understanding that HIIT can induce more time-efficient stimulus to increase aerobic capacity in skeletal muscle,compared with traditional endurance training [21,22]. Therefore, in the present study, we used the HIIT as a type of physical training.SFN was applied as the treatment to the mice that performed an exhaustive exercise test following a 6-weeks HIIT program. With a multi-group design, the purpose of the present study was to investigate the effects of SFN treatment on running distance and duration, muscle Nrf2-mediated antioxidant defense responses, the changes in glutathione (GSH) production, total antioxidant capacity (T-AOC)and 4-hydroxy-2-nonenal (4-HNE) modified protein expression in skeletal muscle, as well as blood creatine kinase (CK) and lactate dehydrogenase (LDH) levels of the mice. We hypothesized that SFN treatment would protect skeletal muscle against the damage induced by exhaustive exercise in the HIIT mice.
2. Materials and methods
2.1 Animals and treatments
The protocols of this study were approved by the Animal Care and Use Committee of Beijing Sport University. Male wild-type (WT)C57BL/6J mice (8 weeks old,n= 24) were purchased from Charles River Development, Inc. (Beijing, China) with an average body weight of (21.43 ± 0.15) g. The animals were housed indoors under a temperature of 20-25 °C, the humidity of 50%-70%, 12 h light/dark cycles, and hadad libitumaccess to deionized water and standard chow. After acclimatization to their housing,the mice were randomly allocated into 3 groups: the control group (CON), pretreated with phosphate-buffered saline (PBS);the HIIT group pretreated with PBS (HIIT); and the HIIT group pretreated with SFN (HIIT+SFN), with 8 mice in each group.Mice were intraperitoneally injected with SFN or PBS 4 times in 3 days (72, 48, 24, and 3 h prior to an exhaustive treadmill test)as described previously [13]. The HIIT + SFN mice were injected 25 mg SFN/kg bw [13,14]. The SFN solution (Shenzhen Fushan Biotech Co., Ltd., China) was diluted with PBS. The CON and HIIT mice were injected PBS at the equal volume of SFN.
2.2 HIIT protocol
There was a 3-day adaptive training session at the beginning of the experiment. During this session, all mice run 5 min (5 m/min)and 10 min (10 m/min) on a treadmill in Day 1, 5 min (10 m/min)and 10 min (15 m/min) in Day 2, and 15 min (15 m/min) in Day 3,respectively. Afterward, the maximal oxygen uptake (VO2max) was determined with a special treadmill: the Oxymax and Metabolic Treadmill (Columbus Instruments, USA) [23]. The VO2max detection was used to determine the exercise intensity (the running speed on the treadmill during HIIT). Briefly, this test started at 10 m/min fo 5 min with an incline of +5° and progressively the speed was increased 3 m/min every 3 min until the mouse spent longer than 10 s on the shock grid without attempting to continue running [24].Mice in the HIIT and HIIT+SFN groups performed a HIIT program as described previously but modified [25], which consisted of 8 series alternating between 2.5 min at an exercise intensity corresponding to about 85% of the VO2max and 2.5 min at an exercise intensity corresponding to about 50% of the VO2max, including 40 min in total. Each session was performed during the morning, three times per week, for 6 weeks.
2.3 Determination of exercise endurance capacity
On the third day after the completion of the 6-weeks HIIT,an exhaustive exercise test on a normal animal treadmill was conducted in all mice and the exercise protocol [23]and the criteria for exhaustion [24]were the same as the VO2max test. Once exhaustion was reached, the power of the shock grid was turned off and the running duration and distance were recorded as the aerobic performance of the mouse. Then, the mice were immediately euthanized by cervical dislocation. The blood sample was gained from the eyeballs of mice. The hind leg muscles were collected, cleaned,and quick-frozen in liquid nitrogen, and then stored at -80 °C.
2.4 Determination of whole blood CK and serum LDH activity
The whole blood CK activity was immediately measured using a Blood CK Measuring Instrument (Reflotron Plus, Roche,Switzerland). Serum LDH activity was determined using the LDH assay kit (A020-2-1; Nanjing Jiancheng Bioengineering Institute,China), following the manufacturer’s protocol. Changes in absorbance were determined with the Bio Tek Synergy H1 (Bio Tek Instruments,Inc., USA) at 450 nm for the LDH activity of lactate to pyruvate conversion assay.
2.5 Real-time quantitative PCR analysis
Total RNA was isolated from crushed gastrocnemius muscles using TRIzol reagent (TaKaRa, Japan), following the manufacturer’s instructions. About 1 μg total RNA was reverse transcribed to cDNA using a kit (FSQ-101; Toyobo Co., Ltd., Japan) according to the manufacturer’s instructions. Moreover, the real-time qPCR was performed in an ABI 7500 Real-time PCR System (Thermo Scientific, Inc., Waltham, MA, USA) using the SYBR Green real-time PCR Master Mix kit (Toyobo Co., Ltd., Osaka, Japan)with the previously synthesized cDNA as the template in a 20 μL reaction volume. Glutamate-cysteine ligase catalytic subunit (Gclc;QT00130543), glutathione reductase (Gsr; QT01758232), catalase(Cat; QT01058106), NAD(P)H quinone oxidoreductase 1 (Nqo1;QT00094367), heme oxygenase 1 (Hmox1; QT00159915), superoxide dismutase 2 (Sod2; QT00161707), glutathione peroxidase1 (Gpx1;QT01195936) and 18S ribosomal RNA (Rn18s; QT02448075)commercial primers from Qiagen (Germany) were used. In addition, the primer sequences ofNrf2, glutamate-cysteine ligase modifier subunit (Gclm)and glutathione synthase (Gss) were listed in Table 1 and these primers were synthesized by Invitrogen Trading (Shanghai, China) Co., Ltd. The difference in gene expression between control and experimental samples was calculated using the 2-ΔΔCtmethod, as described previously [26].

Table 1Mouse real-time quantitative PCR primer sequences.
2.6 Western blotting analysis
Nucleoproteins and total proteins were isolated from about 20 and 25 mg of the gastrocnemius muscles using the nucleoprotein extraction reagents (Solarbio, China) and RIPA protein extraction reagents (P0013B; Beyotime, Inc., China), respectively. The protein concentration was measured using the BCA protein assay kit (Pierce 23225; Thermo Fisher Scientific, Inc., USA).20 μg proteins were separated on Bolt 4%-12% Bis-Tris PlusGels (NW04125BOX; Thermo Fisher Scientific, Inc.) by electrophoresis, and the fractionated proteins were subsequently transferred to a nitrocellulose membrane using iBlot Gel Transfer Stacks Nitrocellulose (IB23001; Thermo Fisher Scientific, Inc.).The blots were probed using the following antibodies: Nrf2(1:1 000, sc-365949; Santa Cruz Biotechnology, USA), 4-HNE-modified protein (1:500, ab46545; Abcam, USA), CAT (1:10 000,66765-1-Ig; Proteintech, China); GPX1 (1:500, ab108427; Abcam,USA), NQO1 (1:500, sc-32793; Santa Cruz Biotechnology, USA),HMOX1 (1:1 000, ab13248; Abcam, USA), SOD2 (1:3 000,sc-30080; Santa Cruz Biotechnology, USA), Histone H1 (1:500,sc-8030; Santa Cruz Biotechnology, USA), and β-actin (1:1 000, sc-47778,Santa Cruz Biotechnology, USA). The density of protein bands was analyzed using Bio-Rad imaging software (Bio-Rad Laboratories,Hercules, CA, USA). The individual values were originally expressed as a ratio of a standard (Histone H1 content, nucleoprotein; β-actin content, total protein) and then expressed as a fold change of the control group value.
2.7 Determination of GSH and T-AOC levels in skeletal muscle
The GSH content was determined from quadriceps femoris by a commercial kit from Nanjing Jiancheng Bioengineering Institute (A061-1-2, Nanjing, China) [27]. The 50 mg tissue was homogenized with 0.4 mL of Reagent 4 in the kit (m/V= 1 g/4 mL) and centrifuged at 3 500 r/min for 15 min at 4 °C to get the supernatants.The total GSH (GSH and GSSG) and GSSG levels in the supernatants were measured by the dithionitrobenzene recycling reaction assay using the plate reader Bio Tek Synergy H1 (Bio Tek Instruments, Inc., USA).GSH level was calculated according to the formula:

T-AOC was assayed in the homogenate supernatant of the gastrocnemius muscle using the commercial assay kit purchased from Solarbio (Beijing, China).
2.8 Determination of muscle glycogen and triglyceride contents
The glycogen and triglyceride contents from quadriceps femoris muscles were determined using the commercial assay kits from Solarbio (BC0345, Beijing, China) and Nanjing Jiancheng Bioengineering Institute (A110-1-1, Nanjing, China), respectively.
2.9 Statistical analysis
All values were reported as the mean ± standard error (SE).Statistical calculations were performed using SPSS STATISTICS v19 software (IBM Corp., USA). Data were analyzed using one-way ANOVA, and a LSD post hoc test was used when significance was found. Significance was set atP< 0.05.
3. Results
3.1 Endurance exercise performance and body weight
The treadmill test showed that the HIIT and HIIT+SFN groups could run longer and farther than the CON group, but the differences with the performance of the CON group were not statistically significant (Figs. 1A and B). However, there was a significant decrease in body weight of the HIIT group, compared to that of the CON group (Fig. 1C).

Fig. 1 Changes of running distance (A), running duration (B) and body weight (C) in the CON, HIIT, and HIIT+SFN groups.
3.2 Whole blood CK and serum LDH levels, muscle glycogen and triglyceride contents, and 4HNE-modified protein expression
The exhaustive exercise elevated whole blood CK and serum LDH levels, as well as the expression of muscle 4HNE-modified protein in the HIIT group, compared with the CON group (Figs. 2A, B and E);however, the HIIT + SFN group had a significantly lower whole blood CK level and the expression of muscle 4HNE-modified protein than those of the HIIT group (Figs. 2A and E). In addition, there were no significant differences in muscle glycogen and triglyceride contents among the groups (Figs. 2C and D).

Fig. 2 Changes of whole blood CK (A) and serum LDH (B) levels, glycogen (C) and triglyceride (D) contents in quadriceps femoris and 4HNE-modified protein expression (E) in gastrocnemius of the CON, HIIT, and HIIT + SFN groups. *P < 0.05, HIIT vs CON group; #P < 0.05, ##P < 0.01, HIIT vs HIIT + SFN group.
3.3 The mRNA expression of Nrf2 and Nrf2-mediated antioxidant genes
There were significant decreases in the mRNA expression ofCat,Hmox1,Nqo1,Sod2andGpx1of skeletal muscle in the HIIT group,compared with those of the CON group (Figs. 3B-3F). Moreover,SFN treatment significantly increased the mRNA expression ofNrf2,Cat, andNqo1in skeletal muscle of the HIIT + SFN group, compared with the HIIT group (Figs. 3A, B and D).

Fig. 3 The mRNA expression changes of Nrf2 (A) and Nrf2-mediated antioxidant genes (B-F) in gastrocnemius of mice. *P < 0.05, **P < 0.01, HIIT vs CON group; #P < 0.05, ##P < 0.01, HIIT vs HIIT+SFN group.
3.4 The expression of Nrf2 nucleoprotein and antioxidant proteins
There were no significant differences in the expression of Nrf2 nucleoprotein and antioxidant proteins (CAT, HMOX1, NQO1,SOD2, and GPX1) between the HIIT and CON groups(Figs. 4A-F).However, the HIIT + SFN treatment significantly increased the expression of Nrf2 nucleoprotein(Fig. 4A) and antioxidant proteins(CAT, HMOX1 and SOD2) of mice skeletal muscle, compared to the HIIT group(Figs. 4B, C and E).

Fig. 4 The expression changes of Nrf2 nucleoprotein (A) and antioxidant proteins (B-F) in gastrocnemius of mice. #P < 0.05, HIIT vs HIIT + SFN group.
3.5 The mRNA expression of key enzyme genes in GSH biosynthesis, GSH and T-AOC levels
The mRNA expression of muscular key enzyme genes in GSH biosynthesis (Gss,Gsr,Gclc, andGclm) and T-AOC level were significantly decreased in the HIIT group, compared with those of the CON groups (Figs. 5A-D and F); while SFN treatment strongly increased muscular mRNA levels of these genes in the HIIT + SFN group, compared with those of the HIIT group (Figs. 5A, C, and D). There was no significant difference in the muscle GSH level among the groups (Fig. 5E).
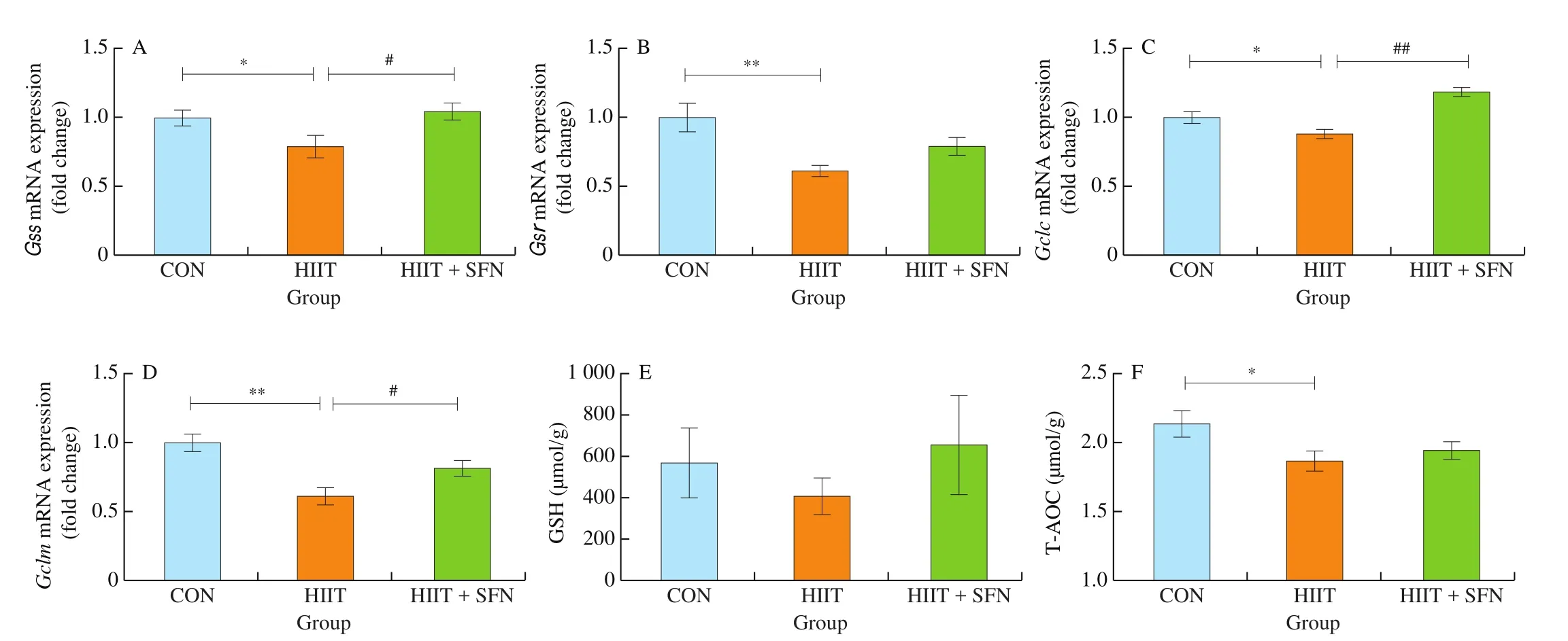
Fig. 5 The mRNA expression changes of key enzyme genes in GSH biosynthesis in gastrocnemius of mice (A-D), GSH (E) and T-AOC (F) levels in quadriceps femoris of mice. *P < 0.05, **P < 0.01, HIIT vs CON group; #P < 0.05, ##P < 0.01, HIIT vs HIIT + SFN group.
4. Discussion
The main findings of the present study revealed that the exhaustive exercise induced a significant increase in whole blood CK, serum LDH and muscle 4-HNE-modified protein expression in the HIIT mice. Moreover, the mRNA expression of some antioxidant genes (Cat,Hmox1,Nqo1,Sod2andGpx1) and key enzyme genes (Gss,Gsr,GclcandGclm) in GSH biosynthesis, as well as T-AOC in skeletal muscle were remarkably lower in the HIIT group than those of the CON mice. SFN treatment showed significant increases in the expression of Nrf2 nucleoprotein, some antioxidant genes/proteins and the genes involved in GSH generation; also,the blood CK and 4-HNE-modified protein levels were remarkably lower in the HIIT-SFN group than those of the HIIT mice. To our knowledge, this is the first study to show that SFN treatment improves the Nrf2-mediated antioxidant defense responses of skeletal muscle induced by exhaustive exercise in the HIIT mice. These results support the hypothesis of the present study.
HIIT protocols are frequently used in modern exercise training and especially in the intermittent sports, such as team or racket sports, to enhance the fitness of the athletes [28]. It is a discontinuous mode of endurance exercise with relatively short bouts of high-intensity workloads interspersed by periods of rest or low-intensity activity. The rationale behind interval training programs is that the totally accumulated time of vigorous exercise is longer than that of the continuous exercise at the same intensity until exhaustion [29].Therefore, it is supposed to yield greater training effects on endurance performance, particularly in highly trained athletes [30]. However, the response and efficiency of various HIIT programs may be different [31],which depends on multiple factors, such as intensity and duration of an interval, number of intervals, number of series, and training level of subjects. Exhaustive exercise increases oxygen consumption and stimulates mitochondria and lymphocytes, which would lead to ROS overproduction [32,33]. ROS can attack the polyunsaturated fatty acid (PUFA) of the phospholipid bilayer on cell membranes [34].4-HNE with a high bioactivity is produced by peroxidation ofω-3 andω-6 unsaturated fatty acid and is the most representative product of lipid peroxidation [35]. When its concentration is higher than normal, it can induce cell apoptosis, influence the cell signal transduction, and have a cytotoxic effect [36]. CK and LDH were at high levels in muscle cells and the breaking of muscle cells releases the two enzymes into the blood stream as muscle damage biomarkers [34,37]. In this study, after the exhaustive treadmill exercise, the HIIT group showed a significant increase in the whole blood CK, serum LDH and muscle 4-HNE-modified protein levels and a significant decrease in the mRNA expression of antioxidant genes and T-AOC level, compared with those of CON group. In addition, there were no significant differences in the expression of Nrf2 nucleoprotein and the measured antioxidant proteins, endurance exercise performance, muscle glycogen and triglyceride contents after the exhaustive treadmill exercise test between the HIIT and CON groups. The data implied that the HIIT protocol did not increase the muscular antioxidative capacity to manage the increased oxidative stress caused by an acute exhaustive exercise test. We also suspect that the current 6-weeks HIIT protocol would be an excessive training load to the mice and they could not adapt to it well. Certainly, more experimental evidence is needed for this idea.
SFN is a specific and potent activator of Nrf2 [11,12]and plays an important role in preventing oxidative stress by activating the Nrf2 signaling pathway [12,14]. Acute exhaustive exercise is a strong stimulus of oxidative stress and a cause of muscle and bone injuries. Previous studies have demonstrated that SFN treatment can protect skeletal muscle against damage induced by exhaustive exercise in untrained rats [14]and the SFN-induced Nrf2 up-regulation and its antioxidative effects in untrained mouse skeletal muscle may play critical roles in attenuating muscle fatigue via reduction of oxidative stress caused by exhaustive exercise [13]. To obtain the further evidence about the effects of SFN treatment on Nrf2-mediated antioxidant defense responses of skeletal muscle induced by exhaustive exercise in exercise trained mice, the 6-weeks HIIT protocol has been adopted in the present study. We found that SFN treatment upregulated the mRNA or protein expression of genes involved in antioxidant and GSH generation in the skeletal muscle, and remarkably decreased the blood CK and muscle 4-HNE-modified protein levels in the HIIT-SFN group than those of the HIIT group. Our observation is consistent with previous studies, although the subjects of these studies were not exercised trainers. Malaguti et al. [14]reported that,in vivo, the oxidative stress caused by exhaustive treadmill exercise was considerably decreased by SFN pretreatment and Angeloni et al. [38]demonstrated that SFN significantly increased total antioxidant activityin vitro. Beside, Nrf2 activation may also play crucial roles in improving lipid and glucose aerobic metabolism by attenuating oxidative stress [3,39,40]; however, in the present study, the exhaustive exercise test did not induce significant differences in the muscle glycogen and triglyceride contents, and endurance exercise performance between the HIIT+SFN and HIIT groups. Therefore, further studies are needed to better elucidate whether a higher SFN dose could have better effects. Nevertheless,our study provides the first direct evidence demonstrating that SFN increased Nrf2-mediated antioxidant defense responses of skeletal muscle induced by exhaustive exercise in the HIIT mice.
There are some limitations in this study. We only focused on the effects of SFN on the mRNA or protein expression of antioxidant enzyme in the skeletal muscle, but did not measure the possible change in their activity. In addition, it is worth to mention, although this study may contribute a better understanding of the protection of SFN against muscle damage during exhaustive exercise in the HIIT mice, whether the 25 mg/kg bw dose used in this study is easily achievable through diet by the consumption of SFN-rich foods remains to be determined. Further studies are needed to define whether SFN oral administration through dietary supplements can exert similar protective effects.
5. Conclusion
SFN treatment improved the antioxidative capacity of skeletal muscle in response to an acute exhaustive exercise,through the increased mRNA and protein expression of Nrf2 and these genes involved in antioxidant and GSH generation, and the decreased blood CK as well as 4-HNE-modified protein levels in the 6-weeks HIIT mice. These findings may provide important implications for athletes.
Declaration of competing interest
The authors declare no conflict of interest.
Funding information
This work was supported by Winter Sports Nutrition Research Center in Beijing Sport University supported by Herbalife NutritionTM;Scientific Research Program Funded by Shaanxi Provincial Education Department (20JK0993 to Y.X.) and Exercise and Physical Fitness, the Key Laboratory of Ministry of Education in Beijing Sport University.
- 食品科學與人類健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species

