Antioxidant effect of Lactobacillus fermentum HFY02-fermented soy milk on D-galactose-induced aging mouse model
Tintin Hu, Rui Chen, Yu Qin, Ke Ye, Xingyo Long,c,Kun-Young Prk,c,*, Xin Zho,*
a Chongqing Collaborative Innovation Center for Functional Food, Chongqing Engineering Research Center of Functional Food,Chongqing Engineering Laboratory for Research and Development of Functional Food, Chongqing University of Education, Chongqing 400067, China
b Department of General Practice, The Third People's Hospital of Chengdu, Chengdu 610036, China
c Department of Food Science and Biotechnology, Cha University, Seongnam 13488, Korea
Keywords:
Lactobacillus fermentum HFY02
Fermented soybean milk
D-galactose
Antioxidant
Aging
A B S T R A C T
This study aimed to investigate the antioxidant effect of soybean milk fermented by a new type of Lactobacillus fermentum (LF-HFY02) by using D-galactose induced aging mice model. Firstly, the optimal fermentation conditions was screened out by detecting the effects of different fermentation temperature and time on the active components and antioxidant activity of soybean milk in vitro. And then unfermented soybean milk and the soybean milk fermented by different Lactobacillus was given by gavage to D-galactose-induced aging mouse. The activities of GSH, GSH-Px, SOD, CAT and T-AOC in serum, brain and liver of soybean milk fermented by LF-HFY02 were significantly increased, while the content of MDA and the level of AGEs in hippocampal were significantly decreased compared with D-galactose induced group. Further more, the mRNA expression of GSH and SOD in mouse liver were obviously up-regulated by soybean milk fermented by LF-HFY02. The skin tissue structure of mice in the LF-HFY02 fermented soybean milk group was more complete, the collagen fibers were increased and arranged orderly and liver inflammation has improved compared with the model group. And Western blot analysis showed that LF-HFY02 effectively upregulated EGFR, SOD and GSH protein expression in mouse liver. These findings suggest that LF-HFY02 can effectively prevent D-galactose-induced oxidation and aging in mice, and the effect was even better than that of the Lactobacillus delbruechii subsp. bulgaricus and vitamin C. Thus, LF-HFY02 may be potentially employed as a probiotic strain. In conclusion, soybean milk fermented by LF-HFY02 can increase the content of antioxidant factors and the activity of antioxidant enzymes by regulating gene and protein expression,and finally inhibit the process of tissue cell peroxidation, and improve the oxidative damage of mouse skin and liver. The results could provide a basis for the research and development and industrial production of probiotic-related fermented soybean milk products.
1. Introduction
Aging is a process closely related to oxidative stress and injury.One mechanism that leads to aging is an increase in free radicals [1,2].A variety of substances with antioxidant activity bothin vivoandin vitrohave become ideal means to prevent aging and objects of anti-aging research [3-6].
Soybean is a cash crop with great nutritional value, and its secondary metabolites (iso flavones, saponins, anthocyanins, phenols,and other components) are beneficial to human health [7,8]. The main components of soybeans are isoflavones, which are very suitable for human health. Iso flavones can be divided into 4 groups(malonylglucoside, acetylglucoside, glucoside, and aglycone) with 3 types (daidzein, genistein, and glycitein), and they have various beneficial effects, such as anti-cancer effects, anti-arteriosclerosis effects, anti-inflammatory effects, estrogenic properties,anti-allergenic effects, lung disease-relieving effects, and so on [9-13].Isoflavones also play a prominent role in antioxidation and have a positive health effect on chronic diseases.
The composition and content of soybean iso flavones in products depend on different processing technologies. Fermentation is an effective, safe, and cheap technology to improve the nutritional quality of soybeans and soybean-related food products, and its effects are beneficial to health [14]. Soy milk is a healthy beverage, and sour soy milk is a fermented soybean product made by microorganisms,which transform soybean isoflavones into active ingredients that can be absorbed by the body more easily, resulting in greater use of the excellent characteristics of soybeans [15]. After fermentation byLactobacillus, mold, or other microorganisms, the antioxidant activity of soy milk increases, and the degree of increase varies with different strains [15-17]. The antioxidant activity of fermented soybean products is significantly higher than that of ordinary nonfermented soybean products [18,19]. Fermented soybeans are a natural food with excellent antioxidants [7].
Manyin vivoandin vitrotests have confirmed that fermented soy milk are good functional natural antioxidants because of their ability to resist oxidative stress and relieve inflammation [7,18,20-24].They also has beneficial health effects, but there are few reports on the antioxidant effect ofLactobacillus-fermented soy milk on aging, especially soy milk that has been fermented by newLactobacillusstrains.
Lactic acid bacteria (LAB) are probiotics with antibacterial,antioxidant, anti-tumor, serum cholesterol-lowering, and immunomodulatory properties. Various LAB, especiallyLactobacillus, have good antioxidant properties bothin vivoandin vitro[19,24]. Probiotic-fermented soy milk is considered to be of great value and can be used as a new type of functional food because it has higher antioxidant activity than unfermented soy milk [25].Lactobacillus-fermented soymilk is a free radical scavenger that can protect pig kidney cells (LLC-PK1 cells) from oxidative damage induced by H2O2by reducing intracellular levels of reactive oxygen species, inhibiting lipid peroxidation, and increasing antioxidant enzyme activity [20].
Traditional fermented food products contain more abundant microbial systems and metabolites than commercial single-strain fermentation products, and also contains some special strains ofLactobacillus[25-30]. Yogurt from Xinjiang, China, is a naturally fermented traditional dairy product that has been popular in the area for more than 10 centuries.L. fermentumis the dominant microorganism in traditional fermented food products [6]. Naturally fermented Xinjiang yogurt is rich in natural LAB, especiallyLactobacillus, which has a variety of physiological activities when it comes to human health. In the early stage of this study,we isolated and identified a new strain ofLactobacillus, namedL. fermentumHFY02(LF-HFY02), from traditional naturally fermented milk from a pastoral area of Xinjiang. We think that natural LAB-fermented soy milk from Xinjiang has good antioxidant efficacy and application potential. Previous studies have shown thatL. plantarum(LP-KSFY02) isolated from fermented milk from Xinjiang can effectively prevent oxidative senescence induced byD-galactose in mice [31]. AnotherL. fermentumstrain(HFY01)can prevent and alleviate dextran sulfate-induced colitis in mice through antioxidation [27]. The aim of this study was to evaluate the antioxidant and anti-aging effects of LF-HFY02-fermented soy milk on mice with aging induced byD-galactose.
2. Material and methods
2.1 Materials and reagents
Soybeans were purchased from Yonghui supermarket in the Nanan District of Chongqing, China. LF-HFY02was selected from naturally fermented yak yogurt from Xinjiang, China, and it was identified by using the National Center for Biotechnology Information’s Basic Local Alignment Search Tool (BLAST).LF-HFY02was stored in the China General Microbial Culture Collection Center (Beijing, China; CGMCC No. 16630). The comparative strain wasL. delbrueckiisubsp.bulgaricus(CGMCC No. 1.16075).
Superoxide dismutase (SOD), glutathione peroxidase (GSH-Px),malondialdehyde (MDA), glutathione (GSH), catalase (CAT) and total antioxidant capacity (T-AOC) kits were purchased from the Nanjing Jiancheng Institute of Biological Engineering (China).Advanced glycation end products (AGEs) kit was purchased from the SenBeiJia Biological Technology Co., Ltd. (China). Vitamin C (VC) andD-galactose were purchased from Shanghai Jingchun Biochemical Technology Co., Ltd. (China).
2.2 Strain activation and fermented soy milk preparation
Probiotics were inoculated into MRS medium according to a 2% seed quantity and cultured at 37 °C for 12 h as the first generation of activation. The bacteria-containing medium after 2 generations of activation was centrifuged, and the bacteria were resuspended with 0.9% normal saline.
The soybeans were then soaked in a soybean-to-water ratio of 1:2 for 12 h and grinded with a soybean-to-water ratio of 1:8.The soy milk residue was filtered with gauze, then sterilized under high pressure. 5 mL soybean milk was inoculated into HFY02 strain (bacterial suspension) according to 2% inoculum amount in a centrifuge tube and fermented at 25, 30, 35 and 40 °C for 0, 3, 6, 9 and 12 h, respectively, to obtain the samples to be tested.
2.3 Extraction and solution preparation of effective components in fermented soybean milk
The soybean milk fermented at different temperatures was pre frozen at -80 °C, and then lyophilized. After that, 5 mL 80% ethanol was added to the lyophilized products to extract the active ingredients.The ultrasonic extraction was performed at room temperature for 6 h. the supernatant was centrifuged at 4 °C for 10 min at 10 000 r/min. Then the extract was placed in the freeze-drying machine for secondary freeze-drying. Weigh about 0.01 g of fermented soybean milk extract, add 5 mL 70% methanol solution to dissolve, prepare 2 mg/mL fermented soybean milk solution, and store it at low temperature for use. See Table 1 of supplementary materials for details.

Table 1Content of soybean iso flavones in each sample.
2.4 Antioxidant determinations by DPPH and ABTS assays
Soybean milk fermented at different temperature for different time were analyzed for free radical scavenging activity in DPPH (1,1-diphenyl-2-picrylhy-drazyl) methanol. The stock solution was prepared by dissolving 24 mg DPPH with 100 mL methanol and then stored at -20 °C until needed. The working solution was obtained by mixing 10 mL stock solution with 45 mL methanol to obtain an absorbance of (1.10 ± 0.02) units at 515 nm using the spectrophotometer. 0.1 mL of methanolic extract and 0.9 mL of fresh DPPH methanol solution (0.1 mmol/L) were mixed. As a control,0.5 mg/mL VC solution was prepared and the same quantity of methanol was used. After dark incubation at room temperature for 30 min, the absorbance was noted at 517 nm. Percent scavenging activity was calculated by the following formula:

The ABTS assay procedure followed the method of Hernández-Ruiz et al. [2]with some modifications. The stock solutions included 7.4 mmol/L ABTS+solution and 2.6 mmol/L potassium persulfate solution. After the mixture of the two stock solutions in equal quantities, the solution was oxidized in the dark for 12 h to form stable free radical ions. The solution was then diluted by mixing 1 mL ABTS+solution with 60 mL methanol to obtain an absorbance of (1.10 ± 0.02) units at 734 nm using the spectrophotometer. Fresh ABTSd+solution was prepared for each assay. Dilute 4 mL mixture for 30 times to obtain ABTS+free radical working solution for use.4 mL working solution and 0.2 mL 0.2 mg/mL fermented soybean milk extract were added into a 5 mL centrifuge tube, mixed and reacted in dark for 30 min. 200 μL of each sample was added to 96 well plate, and measure OD value at 734 nm. The formula of antioxidant activity determination by ABTS was as follows:
Take the average of three parallel samples. Additional dilution was needed if the ABTS value measured was over the linear range of the standard curve.
2.5 High-performance liquid chromatography assay
Six kinds of soybean isoflavones (daidzin, daidzein, genistin,genistein, glycitin, glycitein) were prepared into standard solutions with the concentration of 10, 20, 50, 80 and 100 μg/mL by chromatographic grade methanol. The chromatograms of these 6 kinds of soybean iso flavones were determined by HPLC. Prepare 40 μg/mL mixed solution of 6 kinds of standards, and the chromatogram was determined by the concentration gradient of 1,3, 18, 36 and 40 μg/mL. With the peak area product as the abscissa and the sample content as the ordinate, the standard curve was drawn and the regression equation was calculated. The chromatographic conditions were as follows: Thermo Scientific Accucore C18(4.6 mm × 150 mm, 2.6 μm); mobile phase: A is 0.5% glacial acetic acid aqueous solution, B is acetonitrile; detection wavelength:260 nm; column temperature: 30 °C; flow rate: 0.5 mL/min; injection volume: 10 μL (UltiMate 3000 HPLC (high-performance liquid chromatography) System; Thermo Fisher Scientific, USA). The fermented soybean milk extract solution was used to analyze the change of soybean isoflavone content in the sample; 6 kinds of standard samples and each sample were prepared into a mixture with the concentration of 20 μg/mL and the ratio of 1:7, the spectrum of standard addition sample was measured, and the recovery rate was calculated.
2.6 Animal experiments
Sixty 8-week-old Kunming mice were randomly divided into 6 groups (n= 10): the normal group (N-G), the model group (M-G),the VC group (VC-G), the unfermented soy milk group (USM-G),the LF-HFY02-fermented soy milk group (LFSM-G), and theL. bulgaricus-fermented soy milk group (LBSM-G). The specific operations were as follows. The N-G and M-G groups were fed with 0.2 mL of 0.9% normal saline per day, and the other groups were fed with 0.2 mL of VC (200 mg/kg), 0.2 mL of unfermented soy milk, 0.2 mL ofL. bulgaricus-fermented soy milk, or 0.2 mL of LF-HFY02-fermented soy milk. Starting at the third week, the N-G group was given normal saline as usual, and the other groups received daily abdominal injections of 0.2 mL ofD-galactose (120 mg/kg) in addition to the corresponding reagents. The weight of each mouse was measured every 5 days for 8 weeks. After the last administration,part of the hair on the back of each mouse was removed and the skin tissue was removed after fasting for 12 h. This study was approved by the Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (201906027B, Chongqing, China) and followed the national standard of the People’s Republic of China (GB/T 35892-2018)laboratory animal-guidelines for ethical review of animal welfare. The experimental process was shown in Fig. 1.

Fig. 1 Illustrative diagram of the animal experimental process. USM,unfermented soy milk ; LBSM, L. bulgaricus-fermented soy milk; LFSM,LF-HFY02-fermented soy milk.
2.6.1 Pathological section observation of skin and liver tissues
Skin and liver tissues were removed when the mice were euthanized. The tissues were formalin-fixed, paraffin-embedded,and prepared for histology. The skin samples were stained with hematoxylin and eosin, Masson’s trichrome, and toluidine blue stains with corresponding staining kits (Beyotime, Shanghai, China)according to the manufacturer’s instructions. The sections were then observed under a microscope.
2.6.2 Antioxidant enzyme levels in serum, brain and liver tissue
After measuring the body weight of the mice, blood was collected by eyeball removal in the presence of anticoagulant EDTA. The upper serum used to assess biochemical indexes was collected by centrifuging the whole blood at 4 °C and 3 000 r/min for 10 min.Some brain tissue was homogenized, and the homogenate was centrifuged in a low-temperature environment, then the supernatant was collected. The activity of antioxidant enzymes, such as GSH,GSH-Px, and SOD, and the content of MDA in the serum and brain homogenate were determined according to test kit instructions.
2.6.3 Expression of AGEs in hippocampus
0.2 g of mouse hippocampal tissue was accurately weighed, and 9 times volume of pre cooled normal saline was added according to the ratio of weight/volume = 1/9 (g/mL). The tissue was mechanically homogenized in ice bath. The tissue homogenate was centrifuged at 2 500 r/min for 10 min, and the supernatant was taken for standby.The AGEs in hippocampus was detected by using Mouse AGEs ELISA Kit according to the instructions (Shanghai enzyme linked Biotechnology Co., Ltd., Shanghai, China, ml002154).
2.6.4 Expression of GSH- and SOD-encoding genes in liver tissue
Total RNA was extracted by using RNAzol (Invitrogen, Carlsbad,CA, USA) on ice to keep low temperature environment conditions.The concentration and purity of the RNA were determined by using a microspectrophotometer with 1 μL of RNA and 49 μL of RNase-free water. The appropriate concentration of RNA was reverse transcribed into cDNA according to kit instructions (Tiangen Biotechnology Co., Ltd., Beijing, China). The polymerase chain reaction (PCR)amplification system containing template cDNA and specific gene primers was placed in an octagonal tube and mixed evenly.After brief centrifugation, real-time fluorescence quantitative PCR amplification was performed. The PCR conditions were as follows: denaturation at 95 °C for 15 min, annealing at 60 °C for 1 h, extension at 95 °C for 15 min, and cycling 40 times. With the β-actin-encoding gene as the internal reference,the relative expression of each gene was calculated by using the formula:

The corresponding gene primer sequences was shown in Table 2.

Table 2Primer sequences of RT-qPCR assay.
2.6.5 Western blot assay
The liver tissue was rinsed 3 times with pre-cooled PBS and then homogenized with protein lysate, followed by centrifugation at 10 000 r/min at 4 °C for 15 min. The supernatant fluid was the extracted tissue protein and protein qunatification was performed using a bicinchoninic (BCA) protein quantification kit. The protein samples of each group with the same quality were mixed with the sample buffer and then denatured by heating at 100 °C for 10 min.
The pre-mixed samples and protein ladder were electrophoresed on a PAGE Bis-Tris pre-gel. Then the protein were further transferred onto a PVDF membrane after SDS-PAGE gel electrophoresis.Thereafter, the PVDF membrane was blocked for 1 h with TBST containing 5% skimmed milk, and then incubated in a solution of 5% skimmed milk with a primary antibody including epidermal growth factor receptor (EGFR), GSH, SOD and β-actin at 25 °C for 2 h. Then the PVDF membrane was incubated with a secondary antibody for 1 h at 25 °C after was rinsed 5 times with TBST for 5 min each time. Finally, the enhanced chemiluminescence analysis kit (GE Healthcare, Uppsala, Sweden) and LAS3000 luminescent image analyzer (Fujifilm, Tokyo, Japan) was used to detect and observed the protein expression with β-actin as an internal reference.
2.7 Statistical analysis
All data were expressed as the mean ± standard deviation (SD). The data were analyzedviaone-way ANOVA by using SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA).P< 0.05 was considered significant.
3. Results
3.1 Antioxidant activity in vitro
The antioxidant activity by DPPH and ABTS assays of soybean milk under the same temperature but different fermentation time and the same fermentation time but different fermentation temperature were compared. At the same fermentation temperature, the DPPH radical scavenging rate showed a parabolic trend that gradually increased and then decreased with the extension of fermentation time,and the DPPH scavenging rate of fermented soybean milk was the highest in the range of about 25-30 °C for 9 h (Fig. 2A). At the same fermentation time, DPPH radical scavenging rate also changed with the change of fermentation temperature, but the difference was not particularly obvious (Fig. 2C). The results showed that the DPPH free radical scavenging rate of fermented soybean milk was the highest when fermented at 30 °C for about 9 h.

Fig. 2 In vitro antioxidant properties of soybean milk fermented by LF-HFY02 under different temperature and fermentation time.
Similarly, the scavenging rate of ABTS free radical showed the same trend as that of DPPH. At the same temperature, the scavenging rate was higher when fermented for 6-12 h (Fig. 2B). At the same fermentation time, the scavenging rate of ABTS free radical was the highest when fermented at 30 °C (Fig. 2D). The results showed that the ABTS radical scavenging rate of fermented soybean milk fermented at 30 °C for 6-12 h was the highest.
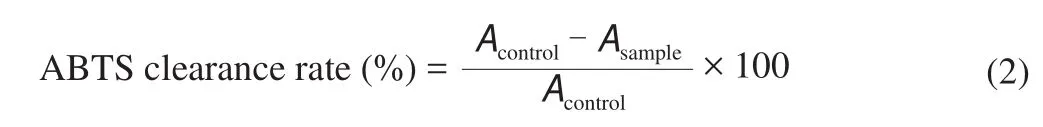
3.2 Content of iso flavone glycosides and iso flavone aglycones in fermented soybean milk
The results showed that the contents of glycitin and glycitein in the samples were less, and the main isoflavones were daidzin,genistin, daidzein and genistein. Through the content analysis of each component, it was found that under the same fermentation time, the higher the temperature, the lower the content of daidzin and genistin (Figs. 3A1and C1); under the same fermentation temperature,the longer the fermentation time, the lower the content of daidzin and genistin (Figs. 3A2and C2). On the contrary, the content of daidzein and genistein in the samples increased with the increase of temperature at the same fermentation time, or with the increase of fermentation time at the same fermentation temperature (Figs. 3B1, D1, B2, D2).

Fig. 3 Analysis of soybean iso flavones in soybean milk fermented by LF-HFY02 under different temperature and fermentation time. (A) Daidzin, (B) daidzein,(C) genistin, and (D) genistein.
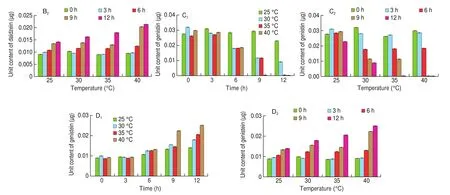
Fig. 3 (Continued)
Among them, the content of daidzein and genistein in the sample fermented at 40 °C for 12 h was the highest (0.021 2 and 0.025 1 μg) compared with the samples without fermentation (0.009 3 and 0.009 1 μg).
3.3 Pathological observation of skin and liver tissues
3.3.1 H&E staining of skin tissue
The results of hematoxylin and eosin staining of skin sections showed that (Fig. 4) in the N-G group, the epidermis was clear and complete, the collagen fibers in the dermis were dense, and the boundary between the dermis and epidermis was clear and neat. In the M-G group, the collagen fibers were loose, the fat vacuoles were increased and exhibited broken voids, and the boundary between the dermis and epidermis was blurred. Compared with the M-G group, the collagen fibers in the VC-G and LFSM-G groups were relatively neat and compact, and the boundary between the epidermis and dermis was clear. However, there were no significant changes in the USM-G and LBSM-G groups.
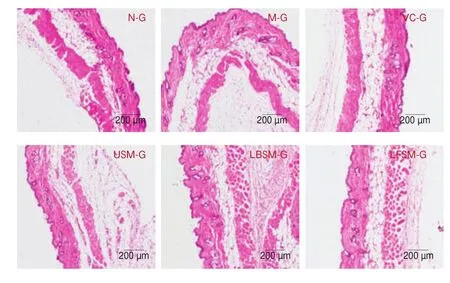
Fig. 4 Hematoxylin and eosin staining pathological observation of mouse skin tissue.
3.3.2 Masson’s trichrome staining of skin tissue
The results of Masson’s trichrome staining of skin sections (Fig. 5)showed that in the M-G group, the dermis became thinner, the content of collagen fibers decreased, and the arrangement of the collagen fibers was loose.

Fig. 5 Masson’s triple staining pathological observation of mouse skin tissue.
3.3.3 Toluidine blue staining of skin tissue
The results of toluidine blue staining of skin sections (Fig. 6)showed that there was a large number of mast cells in the fat layer below the dermis in the M-G group compared with the N-G group.Mast cells decreased significantly in the V-G, LBSM-G, and LFSM-G groups (especially in the LFSM-G).

Fig. 6 Toluidine blue staining pathological observation of mouse skin tissue.
3.3.4 H&E staining of liver tissue
Morphological liver tissue changes are shown in Fig. 7.Compared with the N-G group, the hepatocytes in the M-G group were disordered and irregular, with fuzzy boundaries, swelling, and a large amount of inflammatory in filtration. The pathological changes of hepatocytes in the other groups were alleviated to varying degrees after treatment, but obvious inflammatory in filtration was seen in the USM-G group. The hepatocytes of the LBSM-G, VC-G, and LFSM-G groups were most similar to those of the N-G group, with neat cell arrangement and clear boundaries, relatively neat arrangement of liver cords, less cell in filtration, and clear nuclei. The results showed that LF-HFY02 could reduce the pathological changes of mouse liver tissue induced byD-galactose, and the effect was better than that of unfermented soy milk.
Fig. 7 Hematoxylin and eosin staining of mouse liver tissue.
3.4 Antioxidant activity in serum, brain and liver tissue
As shown in Fig. 8, compared with the N-G group, the MDA content in the serum and brain tissue of the M-G group increased significantly (P< 0.05). The level of serum MDA was reduced significantly in the LFSM-G group (P< 0.05), but there were no statistical differences in the VC-G, USM-G, and LBSM-G groups compared with the N-G and M-G groups. The level of MDA in the brain tissue was reduced significantly in the LFSM-G and LBSM-G groups (P< 0.05). Compared with the N-G group, the levels of GSH,GSH-Px, and SOD in the serum and brain tissue of the M-G group were decreased significantly (P< 0.05), except for GSH-Px in the brain tissue. The levels of GSH-Px in brain tissue and SOD in serum were significantly increased by treatment with LF-HFY02-fermented soy milk, while there were no statistical differences in the other groups in terms of GSH, GSH-Px, and SOD levels.

Fig. 8 Levels of MDA, GSH-PX, GSH, SOD, CAT and T-AOC in the serum, brain and liver tissue of mice. a-d Mean values with different letters in the bar are significantly different (P < 0.05). Values presented are the mean ± SD (n = 8).
3.5 Levels of AGEs in hippocampus tissue
The results (Fig. 9A) showed that the levels of AGEs in hippocampus of aging model mice were significantly higher than those of N-G (P< 0.05). Following treatment with USM, VC, LBSM and LFSM, levels of AGEs were decreased. In particular, the levels of AGEs in mice treated with LFSM were closest to the normal level,and the next were LBSM, USM and VC.

Fig. 9 AGEs in hippocampal tissue and mRNA expression liver tissue of mice. a-d Mean values with different letters in the bar are significantly different (P < 0.05).Values presented are the mean ± SD (n = 8).
3.6 GSH- and SOD-encoding gene expression in liver tissue
The result (Figs. 9B and C) showed that the mRNA expression of the SOD-encoding gene in liver tissue was highest in the N-G group and lowest in the M-G group (P< 0.05). Compared with the M-G group, the level of SOD in the liver tissue of the VC-G, LBSM-G,and LFSM-G groups increased to varying degrees, with the LFSM-G group being closest to the N-G group (P< 0.05). The level of GSH was highest in the LFSM-G group (P< 0.05), but there were no significant differences among the other groups (P> 0.05).
3.7 EGFR, GSH and SOD protein expression in live tissue
Western blot result (Fig. 10) showed that compared with the N-G,protein expression of EGFR, GSH and SOD of liver was significantly down regulated in M-G (P< 0.05). The protein expression in the 4 intervention groups increased compared with the M-G, especially in LBSM-G and LFSM-G, indicating that the expression of EGFR, GSH and SOD proteins could be up regulated by the two kinds of fermented soymilk. Although there was no statistical difference between the two groups, the effect of LSFM was better than that of LBSM.

Fig. 10 Protein expression in mouse liver tissue. a-d Mean values with different letters in the bar are significantly different (P < 0.05). Values presented are the mean ± SD (n = 3).
4. Discussion
Probiotics and their related fermented products are good health food, which have many benefits in improving general health,preventing chronic diseases and prolonging life span [32]. Among many probiotics, lactic acid bacteria play a key role in probiotic fermented food [17]. Fermented soybean products are bean products made by decomposing the substances contained in soybean by microorganisms in the fermentation process. During the fermentation process, some insoluble macromolecular substances in soybean can be transformed into soluble small molecular substances, which can promote the digestion and absorption of soybean products by the intestinal tract; improve the nutritional value of soybean products,reduce allergies and eliminate the soybean which is difficult for most people to accept fishy taste, improve its acceptability, so that the bean products will play its benefits to the extreme [33,34].
In this study, LF-HFY02, which was isolated and identified from natural fermented milk in a pastoral area of Xinjiang, was used as the research object to detect the changes of active components in fermented soybean milk, and further verify and compare its anti-aging effect onD-galactose-induced aging mice.
First of all, the optimal fermentation conditions of soybean milk by LF-HFY02 were screened throughin vitroantioxidative experiments of DPPH and ABTS assays. It was found that the soybean milk fermented at 30 °C for 9-12 h exhibit the best antioxidant propertiesin vitro, and the composition and content of isoflavones in the LF-HFY02 fermented soybean milk changed,which was consistent with many reports [35,36]. The genistein and daidzein content of newly found strainLactobacillus plantarumCQPC01 fermented soybean milk were much more than that ofL. bulgaricus-fermented soybean milk and unfermented soybean milk [36]. Through HPLC analysis, it was found that the content of soybean isoflavones in fermented soybean milk increased with the increase of fermentation time/fermentation temperature, the content of daidzin and genistin decreased while the content of daidzein and genistein increased gradually. The daidzein and genistein accounted for the highest proportion of soybean milk samples fermented at 40 °C for 12 h, while for the lowest proportion of soybean milk samples without fermentation. Genistein, genistin, daidzein and daidzin were soybean isoflavones, which had antioxidant activities [37]. Daidzein played an antioxidant role through antioxidant enzyme system, and can protect tissues from damage [38]. The results showed that LF-HFY02 could convert daidzin and genistin into daidzein and genistein through the fermentation process, so as to improve the antioxidant activity of soybean milkin vitro.
The antioxidant effect of LF-HFY02 fermented soybean milkin vivowas verified byD-galactose aging model. Galactose-induced aging models are ideal models for research on anti-aging treatment interventions because they can simulate the characteristics of natural aging, are simple to operate, and have low mortality [39]. A previous skin histology study of an aging mouse model induced by galactose showed thinner dermal layers, decreased and loosely arranged collagen fibers, degenerated and accumulated elastic fibers, and aging skin in the mice [39].D-galactose is mainly metabolized in the liver. ExcessiveD-galactose leads to the accumulation of galactose and its final metabolite galactosol, which eventually leads to osmotic stress and accumulation of reactive oxygen species through the P-p38 MAPK pathway, which then eventually leads to oxidative stress and the trigger of inflammatory responses [40]. The trigger of inflammatory cascade reactions may lead to a large amount of hepatocyte apoptosis, which damages liver function [41,42]. The skin and liver pathological section results of this study showed significant changes and damage after the induction of aging, which is consistent with reported aging characteristics. LF-HFY02-fermented soy milk significantly improved the abnormal morphology of skin and liver tissues, leading to increased collagen content in the skin, reduced mast cell infiltration, better collagen rearrangement, and improved inflammatory infiltration of the liver, thus showing that LF-HFY02 fermented soy milk could improve skin aging and liver injury induced byD-galactose, followed byL. bulgaricusfermented soybean milk,which was significantly better than that of unfermented soybean milk.
Next, the levels of MDA, GSH-Px, GSH, SOD, CAT, T-AOC in the serum, liver and brain of mice in each group were detected.Among them, MDA was an indicator of oxidative damage and aging,while other indicators can reflect antioxidant capacity. According the literature, theD-galactose-treated animals exhibited increased ROS and MDA levels, decreased antioxidant enzymes such as SOD, CAT and GSH and total antioxidant capacity, suggesting the decreased protection against skin free radicals and the accumulation of lipid peroxidation products [1,43]. It has been con firmed that more than 60 diseases are caused by oxygen free radicals, tissue damage by oxygen free radicals can lead to the production of MDA, and the amount of MDA in the body can indirectly reflect the level of oxidative damage in the body [20].
SOD, CAT, and GSH-Px are groups of antioxidant enzymes that constitute part of a cellular defense system [44]. SOD is an important antioxidant enzyme in organisms, and widely distributed in animals,plants and microorganisms. Studies have shown that superoxide anion free radicals produced by the body can be removed by SOD,thus protecting cells from damage. SOD is an important factor for the body to maintain balance between oxidation and antioxidation [45].Therefore, SOD levels indirectly reflect the antioxidant capacity of the body. Under pathophysiological conditions, reactive oxygen species may cause lipid peroxidation and lead to cell senescence [46,47].GSH-Px is an important peroxidase that can remove lipid peroxides and H2O2, and it plays an important role in preventing oxidation and aging [48]. CAT is a common antioxidant enzyme present in almost all living tissues and responsible for the degradation or reduction of hydrogen peroxide to water and molecular oxygen using either iron or manganese as a cofactor [44]. GSH is an efficient scavenger of oxygen and nitrogen free radicals, and is an important factor to maintain the redox state of cells and homeostasisin vivo. As an antioxidant, GSH reacts with free radicals to form its oxidized form, thus protecting tissues and cells from free radical attack and damage [49]. T-AOC reflects the total antioxidant level of enzyme and non enzyme system.
The results of this study showed that LF-HFY02 fermented soybean milk could significantly reduce the increase of MDA content in serum, liver and brain tissue induced byD-galactose, and increase the levels of GSH-Px, SOD, CAT and T-AOC, and the effect was better thanL. bulgaricusand unfermented groups. These results confirmed that LF-HFY02 fermented soybean milk can regulate antioxidant factors in different tissues to inhibit oxidative damage and prevent oxidative aging.
In addition, the results of GSH and SOD gene expression and protein expression in liver were consistent with the above results,which further proved the antioxidant mechanism of LF-HFY02 fermented soybean milk from different levels.
Except SOD and GSH, the expression of EGFR was also detected in liver. EGFR is widely distributed on the surface of mammalian epithelial cells, fibroblasts, glial cells and keratinocytes. Increased expression of EGFR in aging skin, heart and carcinogenesis [50,51].EGFRs as pro-survival and anti-apoptotic factors age related increase in proliferation and inhibition of apoptosis is considered one of the predisposing factors for development and progression of GI cancers and plays a significant role in modulating these processes in normal,aging and malignant cells [52]. There was a lot of evidence that aging is associated with elevated levels of certain growth factors,particularly EGF-family of peptides, the activation of EGFR plays an important role in the innate immune response of aging mice [53,54]. It was found that LF-HFY02 fermented soybean milk could up regulate the expression of EGFR in liver tissue of aging mice, and the effect was better thanL. bulgaricusfermented soybean milk and unfermented soybean milk.
AGEs are the products of excessive sugar and protein binding.Studies have shown that AGEs can accelerate the aging of human body and lead to many chronic degenerative diseases [55,56]. The increase of its concentration is closely related to aging and oxidative stress, which can be used as a marker of aging. AGEs exist in pyramidal neurons and glial cells. With the development of age and aging, glycosylated beta amyloid protein interacts with AGEs receptors to activate microglia and astrocytes, thus affecting the production of cytokines and cytotoxicity, and promoting the injury of neurons [57]. The results showed that LF-HFY02 fermented soybean milk could significantly reduce the increased content of AGEs in hippocampus of aging model mice, followed by VC andL. bulgaricusfermented soybean milk. It is suggested that the antioxidant effect of the LF-HFY02 fermented soybean milk has a certain role in preventing various neurological diseases caused by aging.
In conclusion, LF-HFY02 fermented soybean milk has excellent antioxidant activityin vivoandin vitro, and the antioxidant mechanism ofLF-HFY02-fermented soy milk may be achieved by increasing glucosidase activity, isoflavone aglycone content, and isoflavone bioavailability. With the hydrolysis of soybean protein,many polypeptide molecular complexes with antioxidant activity are produced gradually. In addition, we have con firmed that LF-HFY02 fermentation changes the composition of glycosides and aglycones in soybean products and the antioxidant capacity of fermented soybean milkin vitro. It has been reported that the activity ofβ-glucosidase with glycosidic ligands and the content of iso flavone aglycones in soy milk are significantly increased by probiotics such asL. acidophilus,L. casei, andBifidobacterium animalis, thereby improving the biological activity of the soy milk (such as its antioxidant effects, its ability to relieve hormone disorders in postmenopausal women, etc.),and there are also changes in the activity of antioxidant enzymes, such as SOD, GSH, and GSH-Px, inLactobacillusfermentation products [40].Probiotic-fermented soy milk can improve the bioavailability of isoflavones, help protein digestion, provide more soluble calcium,enhance intestinal health, and support the immune system. The increased content of iso flavone aglycones in fermented soy milk can improve the biological function of soy milk [16]. In addition, soybean milk fermented by LF-HFY02 may play a final anti-aging role by regulating intestinal microorganisms. Fermented soybean milk can improve the intestinal environment and affect the intestinal and fecal microorganisms [18].
This experiment preliminarily confirmed that LF-HFY02 was a high-quality strain of LAB that LF-HFY02-fermented soy milk could prevent oxidative aging, and the efficacy of LF-HFY02 seemed to be better than that of commercialL. bulgaricus. The experimental data provides a theoretical foundation for further research and development of antioxidant products. However, further development and utilization of LF-HFY02 requires human experiments, the specific mechanism underlying the action of LF-HFY02 in the human body also needs to be explored further.
5. Conclusions
A newL. fermentansnamed LF-HFY02 was discovered by our team in Xinjiang. In this study, we first studied the antioxidant effect of LF-HFY02 fermented soybean milkin vitroand the optimal fermentation conditions. LF-HFY02 fermentation can provide more active soybean isoflavones for soybean milk.LF-HFY02 fermented soybean milk has good antioxidant and anti-aging effects inD-galactose-induced aging mice, and its effect is better than that of LB fermented soybean milk. LF-HFY02 fermented soybean milk can improve the oxidative damage of skin, liver and brain by regulating the expression of antioxidant enzyme gene mRNA and protein expression, regulating the activity of antioxidant enzymes in tissues. LF-HFY02 might be used as a new starter of high quality soybean fermentation functional food. In the future, the mechanism of LF-HFY02 will be strengthened to accumulate theoretical basis for making full use of this resource.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgement
This research was funded by Chongqing University Innovation Research Group Project (CXQTP20033), the Science and Technology Project of Chongqing (cstc2021jcyj-msxmX0408), and Scientific and Technological Innovation Project of Construction of Double City Economic Circle in Chengdu-Chongqing Area of Chongqing Education Commission (KJCX2020052).
- 食品科學與人類健康(英文)的其它文章
- Hypoglycemic natural products with in vivo activities and their mechanisms: a review
- Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health
- Capsular polysaccarides of probiotics and their immunomodulatory roles
- Natural compounds may contribute in preventing SARS-CoV-2 infection: a narrative review
- A comprehensive review on the effects of green tea and its components on the immune function
- A review on current and future advancements for commercialized microalgae species

