Variation of high-molecular-weight glutenin subunits and glutenin macropolymer particle distribution in wheat grains produced under different water regimes
Zhongmin Dai,Yanping Yin,Yong Li,Li Cao,Zhenlin Wang,*
aBiology Department,Dezhou University,Dezhou,Shandong 253023,China
bState Key Laboratory of Crop Biology,Agronomy College,Shandong Agricultural University,Tai'an,Shandong 271018,China
1.Introduction
Wheat(Triticum aestivum L.)is the most widely consumed food crop in the world,being processed to give a range of breads,other baked goods,pasta,and noodles.In wheat,glutenin macropolymers(GMP)are a major component of the grain and an important factor affecting the processing quality of wheat[1].Previous studies demonstrated that the amount of GMP in wheat flour correlates closely with baking quality[2,3].Besides GMP content,GMP particle size and distribution are important in wheat bread-making quality [4].Evidence indicates that GMP particle size strongly correlates with dough development time[5].
GMP consist of high molecular weight glutenin subunits(HMW-GS)linked with low molecular weight glutenin subunits(LMW-GS) through disulfide bonds [6].HMW-GS play an important role in determining the glutenin protein network structure [5],and LMW-GS may also have a specific effect on glutenin aggregation [4].GMP consisting of a higher ratio of HMW-GS to LMW-GS is correlated with improved wheat flour quality[7].Therefore,subunit composition and GMP characteristics determine the rheological properties of wheat dough,and a close correlation between GMP characteristics and end-use quality has been shown.
HMW-GS are encoded by polymorphic genes at the Glu-1 loci on the long arms of group 1 chromosomes.Hexaploid wheat usually contains 3–5 subunits,zero or one encoded by Glu-A1,one or two by Glu-B1 and two by Glu-D1[8].
The content and size distribution of GMP in wheat grains are both genetically and environmentally controlled.Drought promotes HMW-GS accumulation in the early grain filling stage,whereas the opposite effect occurs at late grain filling [9].Increasing N fertilizer increases the proportion of GMP in wheat flour [10].Clay soil results in the accumulation of HMW-GS and GMP when compared to loam soil and sandy soil [11].When under high temperature stress during the kernel filling period,the contents of particular Glu-D1 HMW-GS in weak-gluten wheat are much more sensitive than that in strong-gluten wheat[12].
In recent years,frequent soil water stress in northern China has influenced both dry matter production and quality of wheat[13].Increased N levels promoted the accumulation of HMW and LMW-GS,GMP content and proportion of larger GMP particles under irrigated conditions.Under rainfed conditions,increased N fertilizer also increased protein content [14].Both dough development time and dough stability time were longest with a single post-anthesis irrigation,whereas a second irrigation led to shortened dough development and dough stability times and weakened gluten strength,as well as a decreased glutenin polymerization index and average sized GMP [15].However,information about the impact of different irrigation patterns on accumulations of GMP in wheat grain is still limited.
Although numerous studies have been conducted on size distribution and properties of GMP particles in wheat grains,there is limited information about the size distribution of different quality types of wheat under irrigated and rainfed conditions.The objective of the present study was to investigate differences that may occur in GMP accumulation in field-grown wheat cultivars under irrigated and rainfed regimes.HMW-GS and GMP contents and GMP particle distributions in four wheat cultivars were therefore investigated.
2.Materials and methods
The experiment was conducted on the experimental farm of the Research Institute of Agricultural Science(37°N,116°E),Dezhou,China.Four recently released winter wheat cultivars with different end-use qualities were used.They were Shiluan 02-1(HMW-GS 1Ax1,1Bx7 + 1By9,1Dx5 + 1Dy10)and Jinan 17(1Ax1,1Bx7 + 1By8,1Dx4 + 1Dy12) with strong gluten strength,Yannong 24 (1Ax1,1Bx7 + 1By8,1Dx5 + 1Dy10) with medium gluten strength,Lumai 21 (1Ax1,1Bx7 + 1By8,1Dx5 + 1Dy10)with weak gluten strength.Shiluan 02-1,Yannong 24,and Lumai 21,were used in the growing season of 2010–2011.The 0–20 cm soil layer contained 83.6 mg kg-1of available nitrogen,18.2 mg kg-1of available phosphate and 95.2 mg kg-1of available potassium.Wheat cultivars Jinan 17 and Lumai 21 were used in the 2009–2010 growing season when the soil contained available nitrogen-phosphate-potassium at 81.5,17.6 and 93.6 mg kg-1,respectively.Two contrasting water regimes(irrigated and rainfed) were used.The irrigated treatment was two irrigations with the total water amount of 1500 m3ha-1over the whole growth period(750 m3ha-1each at jointing and booting stages,respectively),whereas the rainfed treatment had no irrigation.The moisture content in soil after anthesis is shown in Fig.1.The experiment was a complete randomized block design with three replicates.Plot dimension was 3 m × 3 m.Plants were sown on 12 October 2010 and 15 October 2009,respectively,at a density of 180 seeds m-2.Normal crop farming practices were implemented to minimize pest,disease and weed incidence.After full heading,spikes flowering on the same date were labeled with thread.At maturity(14 June 2011 and 15 June 2010,respectively),the labeled heads were sampled and used to determine the GMP particle distributions.GMP and HMW-GS contents were also determined.
The content of GMP was analyzed as follows:0.05 g of flour was dispersed into and mixed with 1 mL of SDS and then centrifuged at 15,500 ×g for 15 min using an Allegra X-64R centrifuge(Beckman,San Francisco,CA,USA)and the supernatant was retained.Glutenin macropolymer content was measured using TU-1901 dual-wavelength spectrophotometer(Persee Instruments,Beijing,China).Glutenin macropolymer content was calculated using a set of Kjeldahl protein values.
Glutenin macropolymer-gel was isolated by dispersing 1.4 g of defatted flour in 0.05 mol L-1SDS (pasteurized,28 mL) and then centrifuged at 80,000 ×g for 30 min at 20 °C using a Beckman L-60 ultracentrifuge (Beckman,San Francisco,CA,USA)as described[16].The GMP gel-layer was collected from the top of the supernatant.
For Coulter laser particle size analysis,1 g of GMP-gel was added to 8 mL of 0.05 mol L-1SDS solvent.The tube was sealed and placed on a roller-bank for 3 h at room temperature and analyzed with a Coulter Laser LS13320 (Beckman Coulter Instruments,San Francisco,CA,USA).The GMP surface area distribution and volume distribution were measured and calculated from the resulting pattern.
Quantification of HMW-GS was performed according to the following method[17].In brief,HMW-GS were first separated by SDS-polyacrylate gel electrophoresis (SDS-PAGE) according to Khan et al.[18].A 40 mg grain sample was defatted with chloroform and then mixed with 1 mL of extraction buffer containing 62.5 mmol L-1Tris–HCl (pH 6.8),50% isopropyl alcohol,5% SDS and 1% DTT.The mixture was incubated at room temperature for 30 min with continuous shaking,and then at 60 °C for 1 h,followed by centrifugation at 10,000 ×g for 15 min.The supernatant was used for SDS-PAGE.
The SDS-PAGE gel was 16 cm × 16 cm and 1 mm thick.The acrylamide concentration in the resolving gel was 10%and 4%in the stacking gel.Glutenin extract(20 μL)was loaded in each lane.After electrophoresis,the gel was stained with 0.05%Coomassie Brilliant Blue B250 for 24 h,and then destained in distilled water for 48 h.Thereafter,each band was separately cut from the gel,placed in an Eppendorf tube and depending on the intensity of each band,1 mL of 50% isopropyl alcohol containing 3% SDS was added to the tube which was incubated at 37 °C for 24 h until the gel cleared.The extraction was then monitored at 595 nm with a UV-2401 Shimadzu spectrophotometer(Shimadzu Corporation,Kyoto,Japan).
Analysis of variance was performed with the SPSS statistical analysis package.The statistical model included sources of variation due to genotype,soil water,and genotype × soil water interaction.Data from each sampling date were analyzed separately.Duncan's New Multiple Range Test was employed to assess differences between the treatment means at P = 0.05.General correlation coefficients were calculated between GMP size distribution and contents of GMP and HMW-GS.
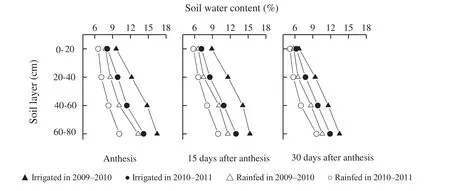
Fig.1-Soil water content(%)after anthesis.▲Irrigated in 2009-2010,●Irrigated in 2010-2011,△Rainfed in 2009-2010,○Rainfed in 2010-2011.
3.Results
Analysis of variance for the percent volume of GMP particles,HMW-GS content and GMP content made it possible to identify the sources of variation(Table 1).Genotype and soil water main effects were significant for these traits except the influence of soil water on the GMP particles of 12–100 μm in 2010–2011.However,genotype × soil water interaction only affected the GMP particles of <12 μm and >100 μm in 2010–2011.This indicated that the interaction was a complicated network.
The contents of total HMW-GS in the four wheat cultivars were ordered as follows: Shiluan 02-1 >Yannong 24 >Lumai 21 in 2010–2011 and Jinan 17 >Lumai 21 in 2009–2010 (Fig.2).Under the rainfed regime,the contents of total HMW-GS increased in all four wheat cultivars.Compared with the irrigated regime,the rainfed regime increased the content of HMW-GS in cultivar Shiluan 02-1 by 3.2%,Jinan 17 by 16.8%(P <0.05),Yannong 24 by 18.5% (P <0.05) and Lumai 21 by 17.0%(P <0.05)in 2009–2010 and 21.8%(P <0.05)in 2010–2011,respectively.This indicated that rainfed conditions increased the content of total HMW-GS in wheat grains,especially in the medium and weak gluten genotypes.
At maturity,cultivars Shiluan 02-1 and Jinan 17 had higher contents of GMP than Yannong 24 and Lumai 21 under both water treatments (Fig.3),indicating that more glutenin was accumulated in the strong gluten genotype than in the medium and weak gluten cultivars.The contents of GMP in the four cultivars showed increasing trends under rainfed conditions with increases of 3.1%,9.3% (P <0.05),10.0% (P <0.05) and 13.8%–18.7%(P <0.05)in Shiluan 02-1,Jinan 17,Yannong 24 and Lumai 21,respectively.
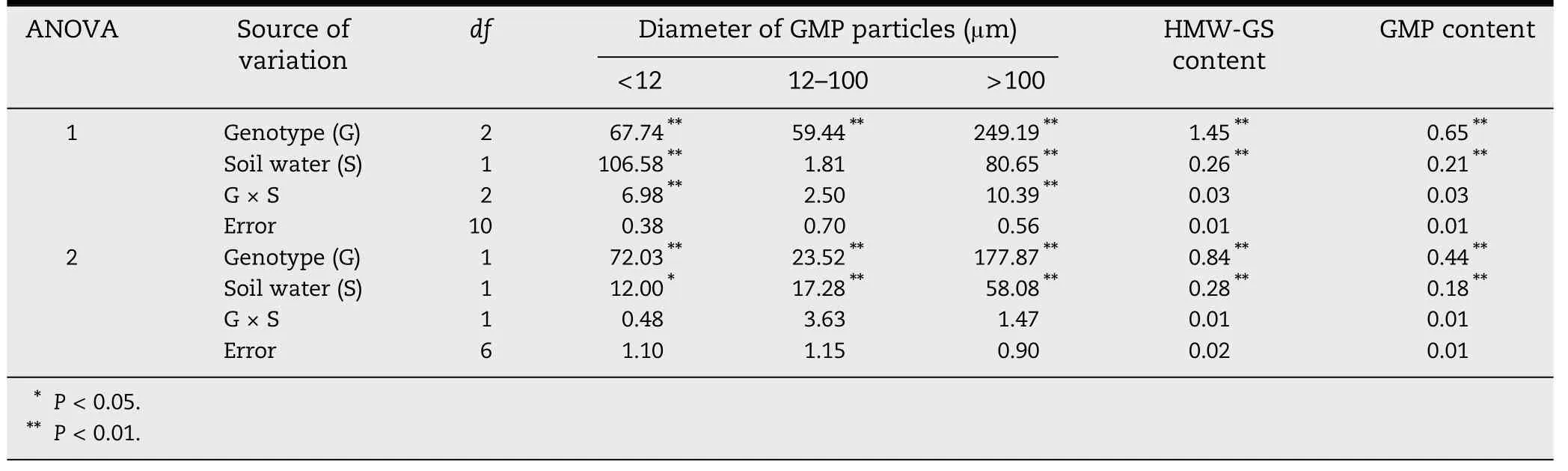
Table 1-Mean squares for treatments and interactions for percent volume of GMP particles and the contents of HMW-GS and GMP in the seasons 2010-2011(ANOVA 1)and 2009-2010(ANOVA 2).
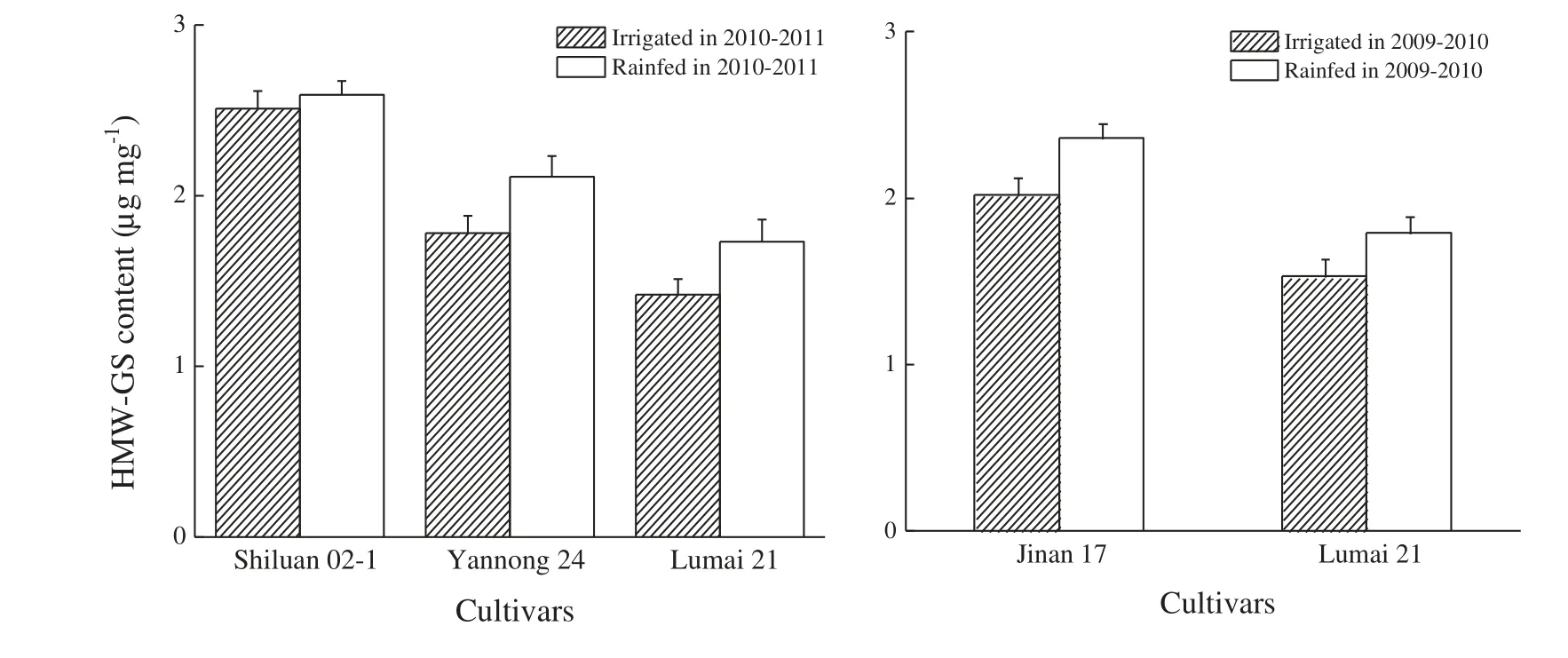
Fig.2-Effect of water treatments on HMW-GS content.
In the four cultivars,the percent volumes of GMP particles with diameters <12,12–100 and >100 μm made up 15.3%–26.1%,47.5%–54.3% and 19.6%–36.2% of the total GMP particles,respectively (Table 2).Under rainfed conditions,the percent volume of particles >100 μm in the four cultivars increased when compared with irrigation,indicating that the rainfed water treatment increased volume percentages of larger particles.
Irrigated and rainfed conditions have different influences on the percent surface area of GMP particles in the four wheat cultivars (Table 3).Compared with irrigation,the percent surface area of >100 μm particles in cultivars Shiluan 02-1,Jinan 17,Yannong 24 and Lumai 21 under rainfed conditions increased by 3.3,12.0,20.8 and 17.6%–50.0%,respectively,indicating that the lower soil moisture promoted increases in the surface areas of large particles in the four wheat cultivars.
The relationships between GMP size distribution and the contents of GMP and HMW-GS are given in Table 4.The GMP and HMW-GS contents were negatively correlated with the percent volume of <12 μm GMP particles (r =-0.756,P <0.05;r =-0.718,P <0.05),but positively correlated to that of >100 μm(r = 0.825,P <0.05;r = 0.806,P <0.05).The result suggested that the large GMP particles have high GMP content.
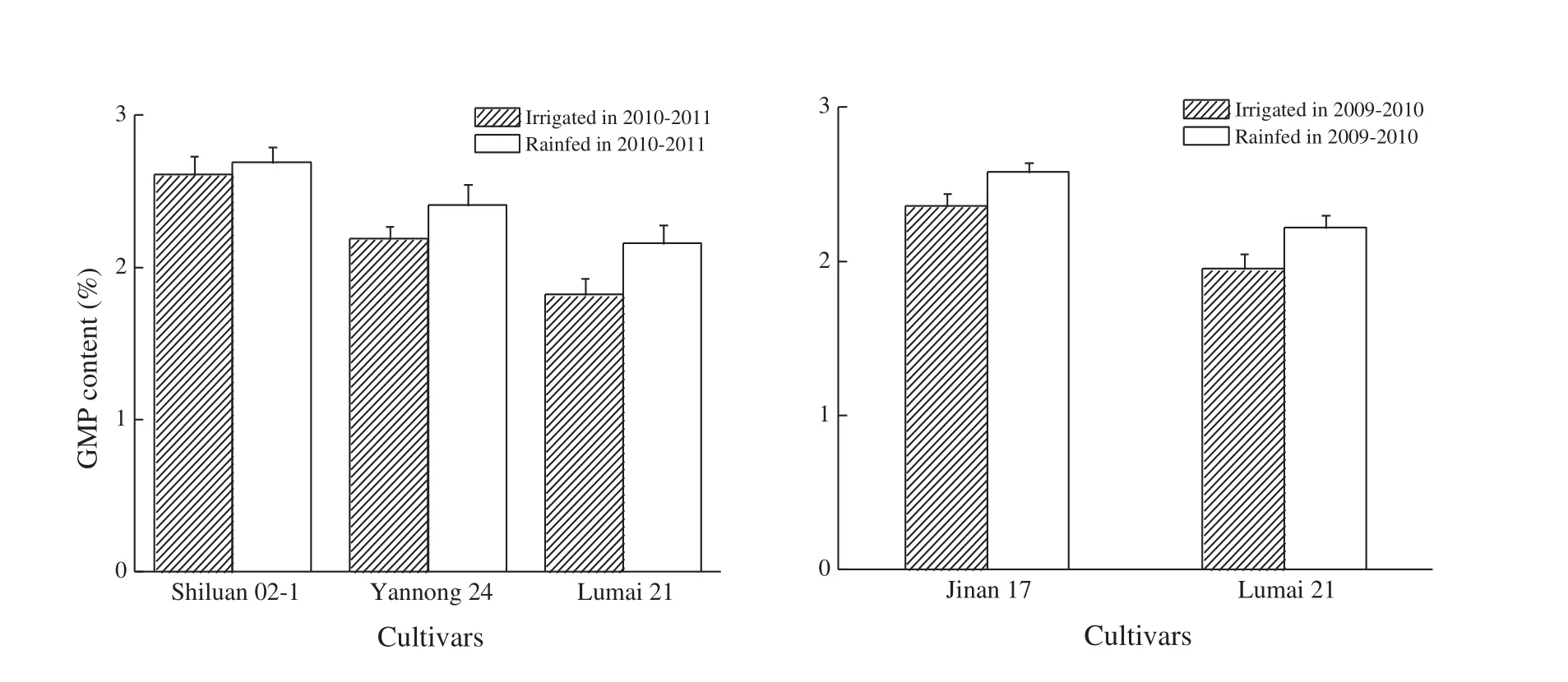
Fig.3-Effect of water treatments on GMP content.
4.Discussion
Analysis of variance showed that genotypes and water treatments significantly affected the size distribution of GMP particles and the contents of HMW-GS and GMP.This infers that water regime has a strong effect on those traits in wheat grains.In the present study,the percent volume and surface area of large particles (>100 μm) under rainfed conditions increased when compared with irrigated conditions,indicating that the different water treatments led to an evident change in the distribution of GMP particles.
GMP consists of spherical glutenin particles and originates from protein bodies in developing grain [19].It was suggested that protein bodies are the building blocks for the formation of much larger glutenin particles formed during the desiccation phase of kernel development[20].A close correlation was found between the accumulation of GMP and the rapid loss of water during desiccation [21].Premature desiccation of the grain induces SDS-insoluble polymer formation,and the percentage of SDS-insoluble polymers as a proportion of total polymers can increase from less than 10%at the end of kernel ripening to 50%in as few as 10 days.In the present study,the percent volume and surface area of large GMP particles under rainfed conditions were markedly increased,suggesting that lower soil moisture is probably beneficial to the desiccation of the grains and thus promotes the formation of large GMP particles.This was also reported by Li et al.[14],who confirmed that rainfed conditions enhance the formation of large GMP particles relative to small ones,resulting in higher GMP volumes and surface area distributions in the wheat grains.
Our data showed that rainfed conditions improved the HMW-GS content and was favorable to the accumulation of GMP large particles,and there was a significant positive correlation between HMW-GS content and percent volume of GMP particles >100 μm (Table 4).It may be concluded that rainfed conditions promote the formation of large GMP particles through enhanced accumulation of HMW-GS.It also confirmed the results of Zhu and Khan [22] showing that environment significantly affected the percentages of total HMW glutenin subunits and individual HMW glutenin subunits from both SDS-soluble and SDS-insoluble glutenin polymers,which in turn affected the size distribution of glutenin polymers.The results indicate that the water regime affected the formation of GMP aggregates by increasing the concentration of HMW-GS.
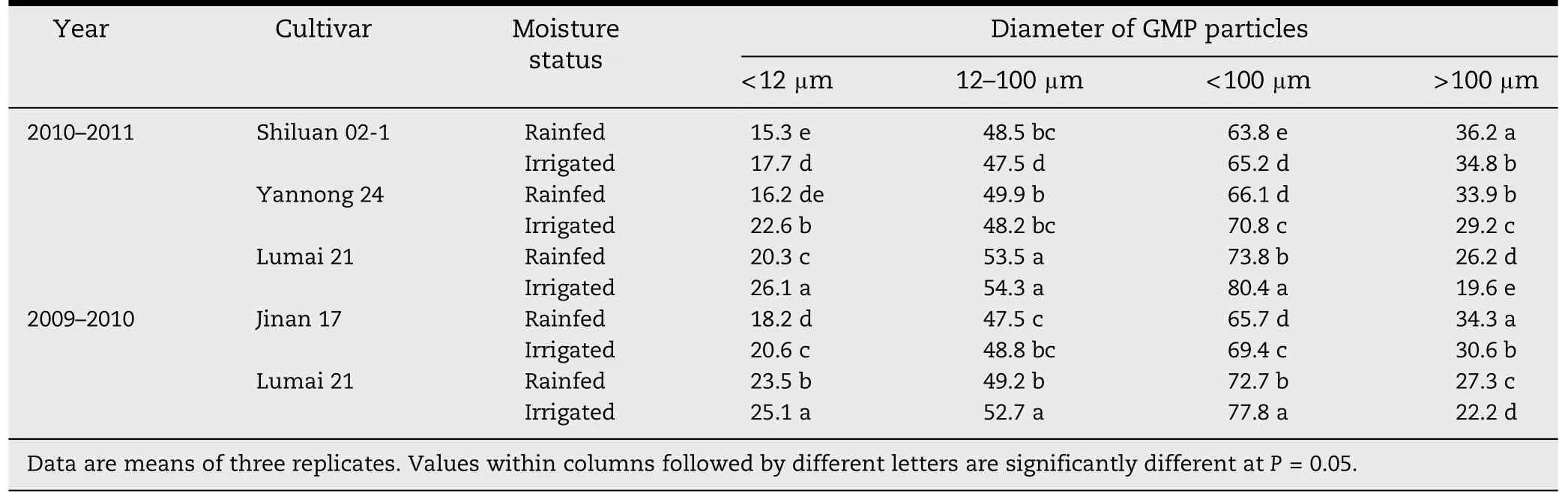
Table 2-Volume distribution of GMP in wheat grains(%).
The content of HMW-GS and GMP,and GMP particle size in cultivars Jinan 17,Yannong 24 and Lumai 21,were increased under rainfed conditions,but the increase in the strong gluten wheat Shiluan 02-1 was less than in the others.Previous studies showed that the subunit pair 1Bx7 + 1By8 was more sensitive to N application and water deficit [14,23].Butow et al.proposed that the 643 bp insertion in the DNA matrix attachment region of 1Bx7 alleles increased transcriptional efficiency [24].This indicates that the subunit components in genotypes may be responsible for the different responses to water treatments.Shiluan 02-1 contained HMW-GS 1Bx7 + 1By9,whereas other wheat cultivars contained 1Bx7 + 1By8.As a result,Shiluan 02-1 was probably less affected by environmental factors than other genotypes.
Compared to irrigated treatment,the rainfed treatment promoted the accumulation of HMW-GS,and increased the proportion of large-size particles of GMP in wheat grains.However,the lower soil moisture also resulted in an apparent reduction in grain yield(data not shown).This is consistent with previous studies that reduced wheat yield under water stress conditions was mainly due to reduction in starch accumulation[25].To manage wheat yield and quality,water treatment should be one of the important factors to be considered.
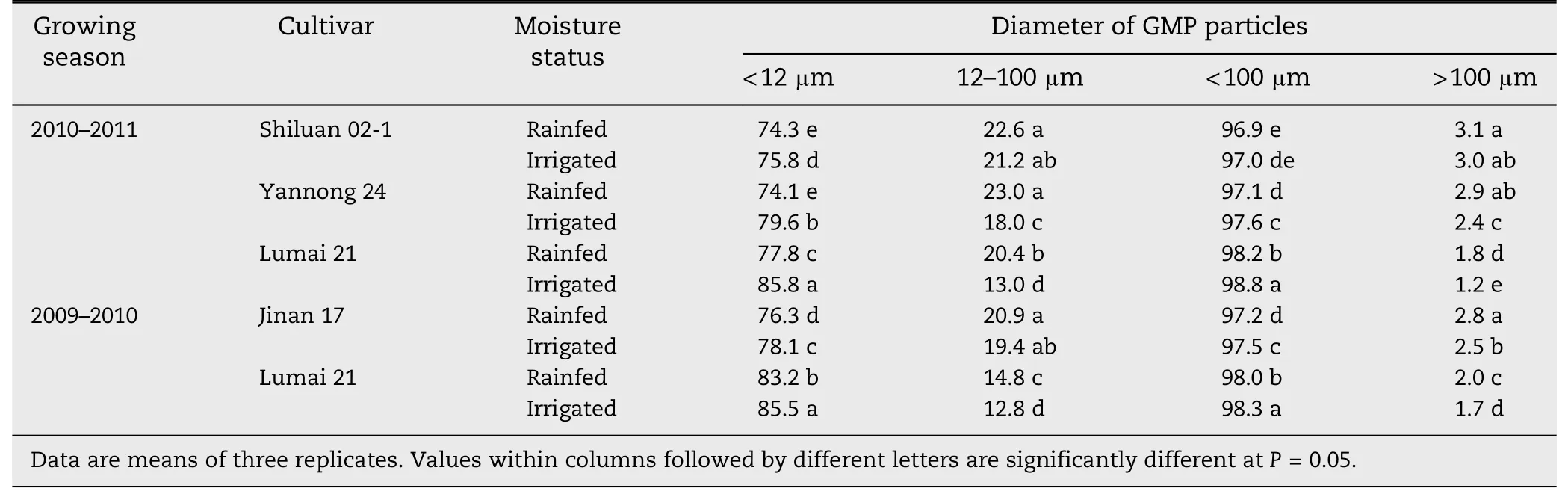
Table 3-Surface area distribution of GMP in wheat grains(%).
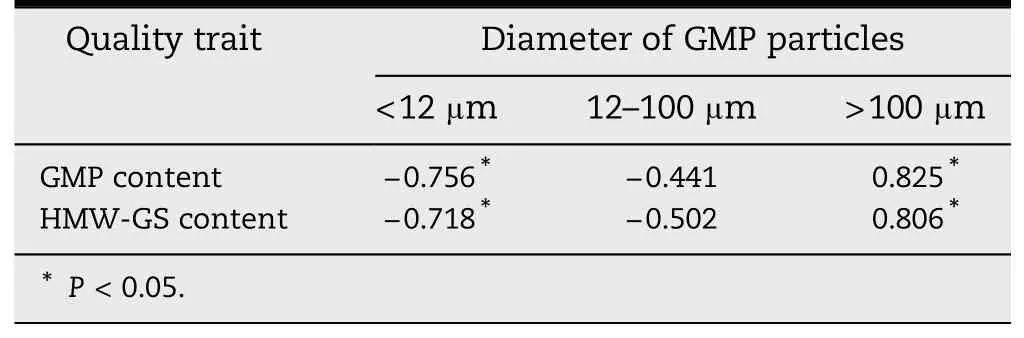
Table 4-Correlation coefficients between GMP particle volume and contents of GMP and HMW-GS.
5.Conclusions
Wheat grain produced under rainfed conditions had higher accumulations of HMW-GS and GMP,and also increased percent volumes and surface areas of large GMP particles,especially in cultivars Yannong 24,Jinan 17 and Lumai 21.This indicates that grain quality was affected by different water regimes.
This research was supported by the National Natural Science Foundation of China (Grant No.31271667),the Natural Science Foundation of Shandong Province,China(Grant No.ZR2010CM044),the National Basic Research Program of China (973 Program,Grant No.2009CB118602),and State Key Laboratory of Crop Biology(Grant No.2012KF01)of Shandong Agricultural University,Tai'an,Shandong,China.
[1] C.Don,G.Lookhart,H.Naeem,F.MacRitchie,R.J.Hamer,Heat stress and genotype affect the gluten in particles of the gluten in macropolymer-gel fraction,J.Cereal Sci.42(2005)69–80.
[2] P.L.Weegels,A.M.van de Pijpekamp,A.Graveland,R.J.Hamer,J.D.Schofield,Depolymerisation and repolymerisation of wheat glutenin during dough processing:I.Relationships between glutenin macropolymer content and quality parameters,J.Agron.Crop Sci.23(1996) 103–111.
[3] A.Tarekegne,M.T.Labuschagne,Relationship between high molecular weight glutenin subunit composition and gluten quality in Ethiopian-grown bread and durum wheat cultivars and lines,J.Agron.Crop Sci.191 (2005) 300–307.
[4] F.M.Dupont,S.B.Altenbach,Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis,J.Cereal Sci.38(2003)133–146.
[5] C.Don,G.Mann,F.Bekes,R.J.Hamer,HMW-GS affects the properties of glutenin particles in GMP and thus flour quality,J.Cereal Sci.44(2006) 127–130.
[6] H.Goesaert,K.Brijs,W.S.Veraverbeke,C.M.Courtin,K.Gebruers,J.A.Delcour,Wheat flour constituents: how they impact bread quality and how to impact their functionality,Trends Food Sci.Tech.16(2005) 12–30.
[7] U.Pechanek,A.Karger,S.Gr?ger,B.Charvat,G.Sch?ggl,T.Lelley,Effect of nitrogen fertilization on quantity of flour protein components,dough properties,and breadmaking quality of wheat,Cereal Chem.74(1997) 800–805.
[8] G.J.Lawrence,P.I.Payne,Detection by gel electrophoresis of oligomers formed by the association of high-molecular-weight glutein protein subunits of wheat endosperm,J.Exp.Bot.34(1983) 254–267.
[9] D.Jiang,H.Yue,B.Wollenweber,W.Tan,H.Mu,Y.Bo,T.Dai,Q.Jing,W.Cao,Effects of post-anthesis drought and waterlogging on accumulation of high-molecular-weight glutenin subunits and glutenin macropolymers content in wheat grain,J.Cereal Sci.195 (2009) 89–97.
[10] E.Triboi,A.Abad,A.Michelena,J.Lloveras,J.L.Ollier,C.Daniel,Environmental effects on the quality of two wheat genotypes:1.Quantitative and qualitative variation of storage proteins,Eur.J.Agron.13 (2000) 47–64.
[11] T.B.Liang,Y.P.Yin,R.G.Cai,S.H.Yan,W.Y.Li,Q.H.Geng,P.Wang,Y.H.Wu,Y.Li,Z.L.Wang,HMW-GS accumulation and GMP size distribution in grains of Shannong 12 grown in different soil conditions,Acta Agron.Sin.34(2008)2160–2167,(in Chinese with English abstract).
[12] Z.Deng,J.Tian,L.Zhao,Y.Zhang,C.Sun,High temperature-induced changes in high molecular weight glutenin subunits of Chinese winter wheat and its influences on the texture of Chinese noodles,J.Agron.Crop Sci.194(2008) 262–269.
[13] R.K.Ma,X.L.Jia,Q.G.Zhang,L.H.Zhang,Y.R.Yao,L.H.Yang,Physiological characteristics of water in wheat cultivar SX733: the effect of water saving irrigation,Acta Agron.Sin.33(2007) 1446–1451,(in Chinese with English abstract).
[14] Y.Li,Y.P.Yin,Q.Zhao,Z.L.Wang,Changes of glutenin subunits due to water–nitrogen interaction influence size and distribution of glutenin macropolymer particles and flour quality,Crop Sci.51 (2011) 2809–2819.
[15] F.J.Yao,M.R.He,D.Y.Jia,X.L.Dai,Q.Cao,Effects of post-anthesis irrigation on degree of polymerization of storage protein and rheological properties in wheat,Cnin.J Plant Ecol.34(2010)271–278,(in Chinese with English abstract).
[16] A.Graveland,P.Bosveld,W.J.Lichtendonk,J.P.Marseilles,J.H.E.Moonen,A.Scheepstra,A model for the molecular structure of the glutenin from wheat flour,J.Cereal Sci.3(1985)1–16.
[17] R.Q.Liang,Y.R.Zhang,M.S.You,S.F.Mao,J.M.Song,G.T.Liu,Multi-stacking SDS-PAGE for wheat glutenin polymer and its relation to bread-making quality,Acta Agron.Sin.28 (2002)609–614,(in Chinese with English abstract).
[18] K.Khan,R.Frohberg,T.Olson,L.Huckle,Inheritance of gluten protein components of high-protein hard red spring wheat lines derived from Triticum turgidum var.dicoccoides,Cereal Chem.66(1989) 397–401.
[19] C.Don,W.J.Lichtendonk,J.J.Plijter,R.J.Hamer,Glutenin macropolymer: a gel formed by particles,J.Cereal Sci.37(2003) 1–7.
[20] T.W.J.M.Van Herpen,J.H.G.Cordewener,H.J.Klok,J.Freeman,A.H.P.America,D.Bosch,M.J.M.Smulders,L.J.W.J.Gilissen,P.R.Shewry,R.J.Hamer,The origin and early development of wheat glutenin particles,J.Cereal Sci.48(2008) 870–877.
[21] J.L.Carceller,T.Aussenac,Size characterisation of glutenin polymers by HPSEC-MALLS,J.Cereal Sci.33(2001) 131–142.
[22] J.Zhu,K.Khan,Quantitative variation of HMW glutenin subunits from hard red spring wheats grown in different environments,Cereal Chem.79 (2002) 783–786.
[23] H.Wieser,G.Zimmermann,Importance of amounts and proportions of high molecular weight subunits of glutenin for wheat quality,Eur.Food Res.Technol.210 (2000) 324–330.
[24] B.J.Butow,B.W.Ma,K.R.Gale,G.B.Cornish,L.Rampling,O.Larroque,M.K.Morrell,F.Bekes,Molecular discrimination of Bx7 alleles demonstrates that a highly expressed high-molecular weight glutenin allele has a major impact on wheat flour dough strength,Theor.Appl.Genet.107 (2003)1524–1532.
[25] A.Ahmadi,D.A.Baker,The effect of water stress on the activities of key regulatory enzymes of the sucrose to starch pathway in wheat,Plant Growth Regul.35 (2001)81–91.
- The Crop Journal的其它文章
- Identification and fine mapping of two blast resistance genes in rice cultivar 93-11
- Zea mays(L.) P1 locus for cob glume color identified as a post-domestication selection target with an effect ontemperate maize genomes
- Identification of unconditional and conditional QTL for oil,protein and starch content in maize
- Effects of narrow plant spacing on root distribution and physiological nitrogen use efficiency in summer maize
- Dissection of two quantitative trait loci for grain weight linked in repulsion on the long arm of chromosome 1 of rice(Oryza sativa L.)
- Genome-wide association of 10 horticultural traits with expressed sequence tag-derived SNP markers in a collection of lettuce lines

