煅燒溫度對花朵狀鎢酸鉍光催化活性的影響
盛珈怡 李曉金 許宜銘
(浙江大學化學系,硅材料國家重點實驗室,杭州310027)
1 Introduction
In the recent years,the photocatalysis of compounds containing Bi has been widely investigated,1-3among which Bi2WO6has attracted much attention.4,5This solid has small band gap energy of 2.8 eV falling within the solar spectrum,and can initiate redox reactions for organic degradation in aerated aqueous solution under either UV or visible light.Early in 1999,Kudo and Hijii6found that Bi2WO6was active under visible light for O2evolution from AgNO3solution,and for H2evolution from methanol solution,respectively.This catalyst was prepared via a conventional solid state reaction,and thus it had a small surface area of 0.2 m2·g-1.Since then,different Bi2WO6solids with a high surface area and photocatalytic activity have been reported,including nanocrystals,7nanocages,8nanofibrous mat,9and flower-like pallets.10,11Moreover,modifications of Bi2WO6with F,12,13Fe,14Pt,15Mo16-18resulting in an enhanced photocatalytic activity has been also claimed.However,information on factors influencing the photocatalytic activity of pristine Bi2WO6is very limited.For example,the optimum preparation temperature that corresponds to the maximum activity of Bi2WO6is different from one report to another.When Bi2WO6was hydrothermally synthesized at 100-220°C,such optimum temperature was 160°C for acetic acid photodecomposition,19but it was 180-200°C for Rhodamine B(RhB)photobleaching.20,21Besides,when Bi2WO6was thermally treated at 400-700°C,its photocatalytic activity for acetic acid decomposition increased with increasing the temperature.22It is highly possible that both the crystallinity19and surface area22of Bi2WO6are important influencing factors.
In this work,we investigated the effect of sintering temperature on the physical properties and the optimum sintering temperature of flower-like Bi2WO6,and the factor of surface area is also considered.Sample was prepared from a hydrothermal reaction at 160 °C,followed by annealing in air at 300-600 °C for 3 h.In order to minimize possible effect of dye sensitization and organic adsorption on the activity assessment,colorless and poorly adsorptive phenol was used as a model substrate.Solid was characterized by several techniques.The observed sintering temperature dependent activity of Bi2WO6is discussed,in terms of the crystalline structure,surface area,light absorption,and defect sites.
2 Experimental
2.1 Materials
All reagents were used as received without further purification.Na2WO4·2H2O(AR,99.5%)and phenol(AR,99.5%)were purchased from Sinopharm Chemical Reagent Co.,Ltd.Bi(NO3)3·5H2O(AR,99.0%)was purchased from Aladdin Chemistry Co.,Ltd.HNO3(AR,65%)was purchased from Zhejiang Zhongxing Chemical Reagent Co.,Ltd.
2.2 Synthesis of Bi2WO6
Sample was synthesized by a modified hydrothermal method.10,11Typically,1.26 g of Na2WO4·2H2O dissolved in 40 mL of water was added dropwise to the solution containing 3.64 g of Bi(NO3)3·5H2O and 30 mL of 0.4 mol·L-1HNO3.The suspension was sonicated for 30 min,transferred into a 150-mL Teflon-lined autoclave,and heated at 160°C for 20 h.After cooling to room temperature,the particles were collected by centrifugation,washed with water,and dried at 80°C overnight.Finally,the sample was sintered at different temperatures for 3 h.
2.3 Solid characterization
Scanning electron microscope(SEM)was performed on a Hitachi S-4800.Photoluminescence(PL)spectra were recorded at room temperature on a Shimadzu F-2500 spectrophotometer,and the excitation wavelength was 330 nm.X-ray diffraction(XRD)patterns were recorded on a D/max-2550/PC diffractometer(Rigaku).According to the strongest diffraction at 2θ=28.3°,the average crystallite size(ds)of Bi2WO6was calculated by using the Scherrer equation.Raman spectra were obtained on a Jobin Yvon Lab Ram 1B with 632.8 nm He-Ne laser excitation.Nitrogen adsorption was measured at 77 K on a Micromeritics ASAP2020 apparatus,from which the Brunauer-Emmett-Teller(BET)surface area and total pore volume(Vt)were calculated.Diffuse reflectance spectra(DRS)were recorded on a Varian Carry 500 using BaSO4as a reference,and the reflectance(R)was transformed to the Kubelka-Munk FR=(1-R)2/2R.The band gap energy(Eg)of Bi2WO6was estimated by a derivative method.23These physical parameters of Bi2WO6are tabulated in Table 1.
2.4 Photocatalytic experiment
Reactor was made of Pyrex glass,and thermostated at 25°C through a water-cycling jacket.The suspension containing 1.30 g·L-1of catalyst,and 0.43 mmol·L-1of phenol was first stirred in dark for 1 h,and then irradiated with a high pressure mercury lamp(300 W).At given intervals,small aliquots were taken,filtered(0.22 μm),and analyzed on a Dionex P680 high performance liquid chromatography.
3 Results and discussion
3.1 Characterization
Fig.1 shows the SEM images of Bi2WO6samples sintered at different temperatures(Ts).The as-prepared sample was flower-like,with a diameter ranging from 1 to 6 μm.This superstructure was constructed from a number of nanoplates with a length from 400 nm to 1 μm.After thermal treatment at 350 °C,the nanoplates became large in size.Then,they transformed to various nanorods and nanobricks at 500 and 600°C,respectively.XRD patterns and Raman spectra showed that all samples were in the crystal form of orthorhombic Bi2WO6without other phases.The diffraction patterns in Fig.2(A)could be indexed to pure orthorhombic Bi2WO6(PDF No.39-0256).The refined cell volume((0.4880±0.0007)cm3)and lattice parameters(a=(0.5455±0.0003)nm,b=(1.6436±0.0024)nm,and c=(0.5444±0.0005)nm)were similar to those reported.6-11Raman spectra in Fig.2(B)displayed several vibrations in 50-1000 cm-1,characteristics of orthorhombic and plate-like Bi2WO6.24,25Moreover,with the increase of Ts,both XRD and Raman peaks became more and more intensive and acute,indicative of the growth of Bi2WO6nanocrystals.By application of the Scherrer equation,the average crystallite size of Bi2WO6along the(131)direction was calculated,which increased indeed with increasing Ts(Table 1).Meanwhile,the BET surface area and pore volume of Bi2WO6also decreased with the sintering temperature Ts(Table 1).Since N2adsorption mainly occurs on the external surface of solid,these observations indicate that upon the thermal treatment,Bi2WO6nanoplates not only grow to large crystals,but also sinter together to large aggregates with a damaged porous structure.
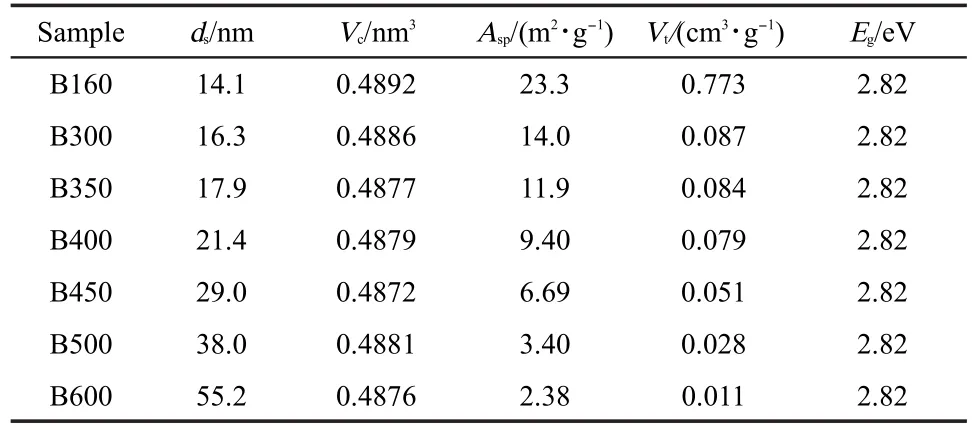
Table 1 Physical parameters of Bi2WO6samples sintered at different temperatures
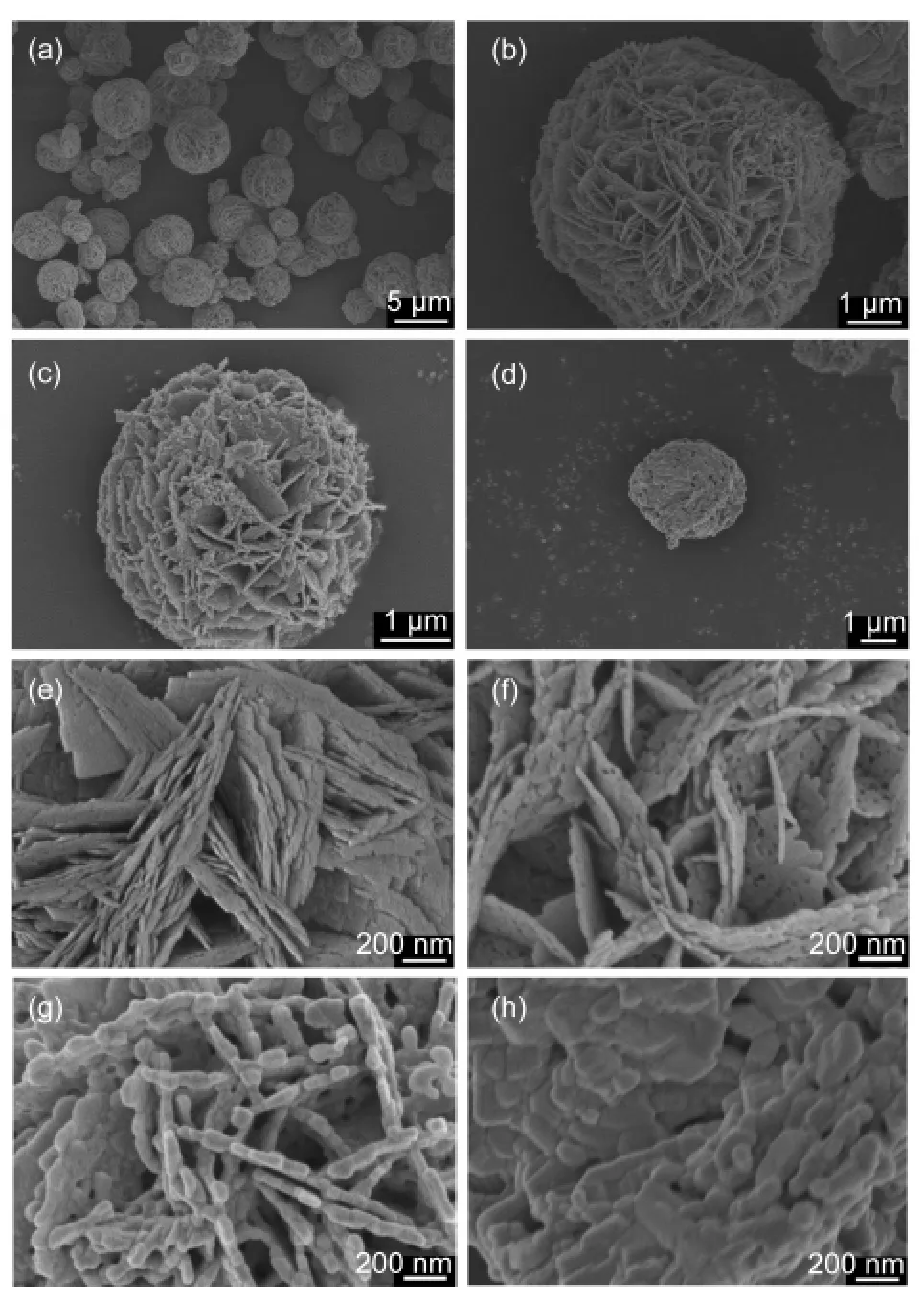
Fig.1 SEM images of Bi2WO6samples sintered at(a,b,e)160°C,(c,f)350 °C,(g)500 °C,and(d,h)600 °C
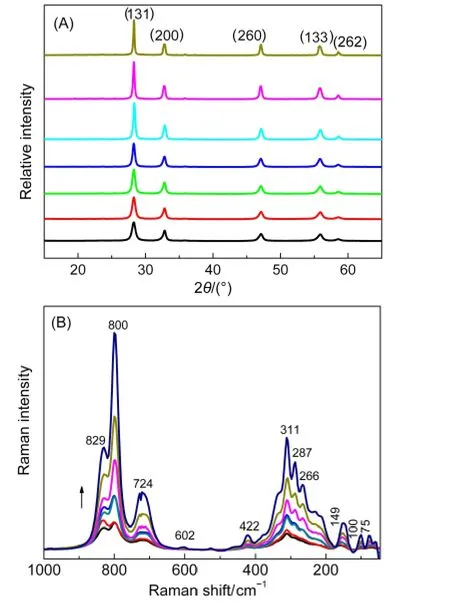
Fig.2 (A)XRD patterns and(B)Raman spectra of Bi2WO6 samples sintered at different temperatures
Fig.3 shows the diffuse reflectance transformed absorption and PL spectra of Bi2WO6sintered at 350°C.There was a broad absorption band at 200-450 nm,ascribed to the charge transfer from O2-to W6+.By using a derivative method,23the band gap energy(Eg)of Bi2WO6was estimated to be 2.82 eV(or 440 nm),very close to those reported.6-11In the PL spectra,the emission at 450 nm is attributed to the band-to-band transition,while other peaks at 470,482,492,and 518 nm are assigned to the radiative recombination of charge carriers mediated by surface states.26Furthermore,all samples showed no differences either in Eg(Table 1)or in the shape and peak position of PL spectrum.These observations suggest that all samples of Bi2WO6have similar electronic band gap energies and kinds of surface states.
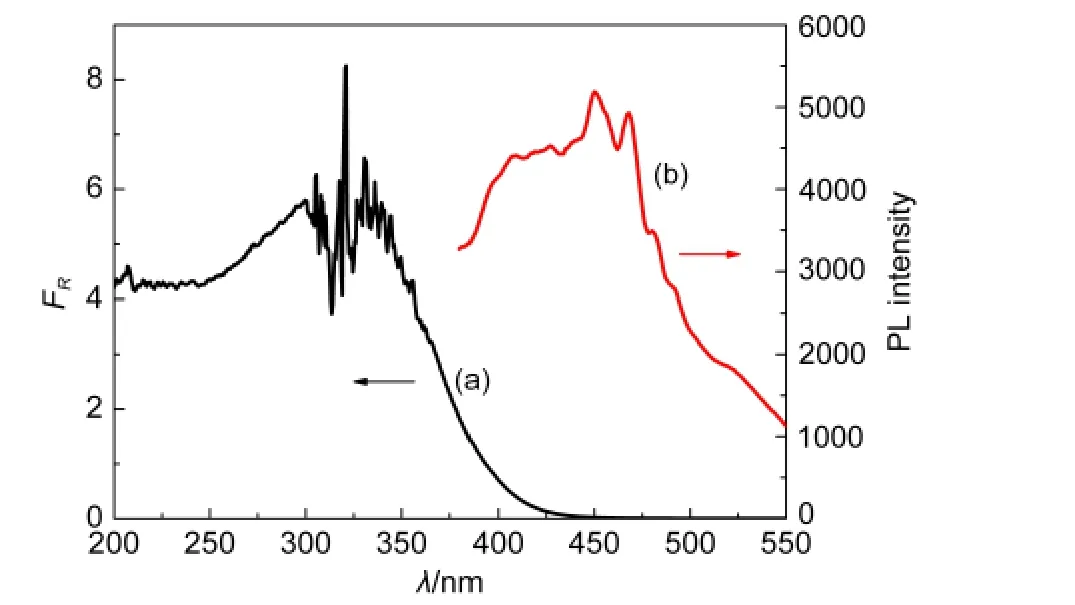
Fig.3 (a)Diffuse reflectance transformed absorption spectrum,and(b)PLemission spectrum of Bi2WO6sintered at 350°C
3.2 Photocatalytic activity
Fig.4(A)shows the time profiles of phenol degradation on Bi2WO6sintered at 350°C.Under UV light,phenol concentration decreased with time,and the kinetics well fitted to the pseudo-first-order rate equation.However,the rate constant of phenol degradation obtained with Bi2WO6(kobs=2.35×10-3min-1)was approximately one order of magnitude lower than that measured with P25 TiO2(kobs=1.97×10-2min-1).Similar trend in the activity was also observed for the formation of hydroquinone and benzoquinone from phenol degradation(open symbols in Fig.4(A)).Moreover,with Bi2WO6or TiO2,total amount of organic intermediates produced was approximately 10%of total phenol degraded.These observations confirm that Bi2WO6can initiate organic degradation and mineralization,like TiO2.Control tests in the dark or under UV light without catalyst showed negligible phenol degradation.
Fig.4(B)shows the apparent rate constants of phenol degradation obtained with different catalysts under similar conditions.Since phenol adsorption on each catalyst in aqueous solution was negligible,this rate constant could be taken as a measure of the relative photoactivity among the catalysts.With the increase of Ts,the photocatalytic activity of Bi2WO6first increased,and then decreased.A maximum activity of Bi2WO6was observed at 350°C,which is notably different from those reported.19-22This discrepancy may result from the effects of surface area,dye sensitization,organic photolysis,and adsorption on the activity determination.
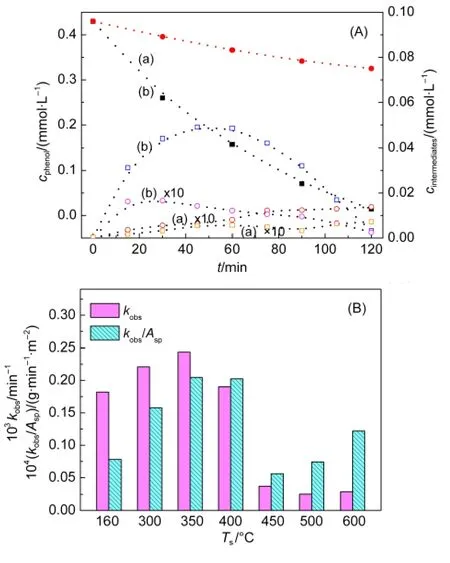
Fig.4 (A)Time profiles of phenol degradation(solid bars)over(a)Bi2WO6sintered at 350°C,and(b)P25 TiO2,and the corresponding formation of hydroquinone(open square bars)and benzoquinone(open circle bars).(B)Apparent rate constant(kobs)and surface area-normalized rate constants(kobs/Asp)of phenol degradation over Bi2WO6sintered at different temperatures
Considering that the catalysts have different surface areas,the apparent rate constant of phenol degradation was then normalized with the surface area obtained by N2adsorption(Table 1),and result is shown in Fig.4(B).This specific rate(kobs/Asp)as a function of Tswas different from that trend in kobs.But,the maximum value of kobs/Aspwas still located at Ts=350°C.Interestingly,in the region of Tsfrom 450 to 600°C,the specific rate increases with Ts,which is similar to the apparent rate of CO2production increasing with Ts,observed with acetic acid photodecomposition on flower-like Bi2WO6.22However,the actual surface area for the particles suspended in water is not known,which would be different from that measured by N2adsorption.Therefore,this plot of kobs/Aspagainst Tsis only useful as a reference.
3.3 Possible explanation
Amano and coworkers22have recently reported that the crystalline content of Bi2WO6is an important factor determining its activity for the photogeneration of conduction band electron,and for the photodecomposition of acetic acid.In the present study,the crystalline content of Bi2WO6also increases with the increase of Ts(Fig.1).However,such trend in the crystalline content is not always consistent with that in the activity for phenol degradation(Fig.4(B)).In other words,the photocatalytic activity of Bi2WO6is not only determined by its crystallinity,but also by some other factors.
In fact,there was a notable difference in the absorption and emission intensities among the samples.Since the photoreaction was conducted at λ≥320 nm,the absorbance at wavelengths between 320 and 450 nm was then integrated,and presumably used as a measure of light intensity absorbed by Bi2WO6in the reactor.Fig.5(A)shows the integrated absorbance at 320-450 nm,and the band-to-band emission at 450 nm.The maximum absorbance and the minimum emission were located at 300 and 350°C,respectively.The absorbance changing with Tsmay result from the interplay between the morphology and particle size of flower-like Bi2WO6.22In general,a weaker intrinsic emission represents a lower recombination of charge carriers,and a higher efficiency for surface reaction.However,neither the absorbance nor the band-to-band emission intensity as a function of Tsexactly matches the trend in the photocatalytic activity for phenol degradation(Fig.4(B)).It appears that the Ts-dependent photoactivity of Bi2WO6is due to the combined effects of light absorption and surface defects.Through normalization with the maximum emission at 450 nm for each sample,the emission peaks due to surface states were observed to increase,and decrease with Ts(Fig.5(B)).A maximum number of surface defects were located at 350°C,matching well the maximum photocatalytic activity of Bi2WO6at 350°C(Fig.4(B)).These surface states may serve as traps for the photogenerated charge carriers,consequently improving the efficiency of charge separation and accelerating reactions at the solid-liquid interface.
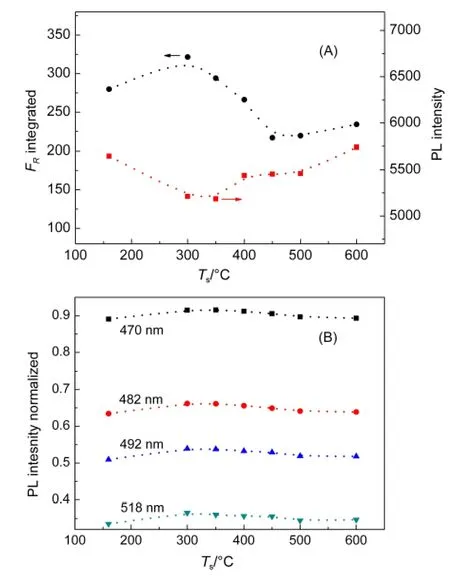
Fig.5 (A)Integrated FRat 320-450 nm and the PLemission at 450 nm;(B)the normalized emission intensity at different wavelengths as indicated by the legends
4 Conclusions
In this work,the effect of sintering temperature on the photocatalytic activity of Bi2WO6has been examined by using phenol as a model organic substrate.The optimum sintering temperature that corresponds to the maximum activity of flowerlike Bi2WO6is 350°C,much different from those reported.The observed Ts-dependent photoactivity of Bi2WO6is attributed to the combined effects of crystallinity,light absorption,and surface defects.This information on the Tseffect would be useful for further development of a highly photoactive Bi2WO6.
(1) Zhang,A.P.;Zhang,J.Z.Acta Phys.-Chim.Sin.2010,26,1337.[張愛平,張進治.物理化學學報,2010,26,1337.]doi:10.3866/PKU.WHXB20100533
(2) Lin,X.;Lü,P.;Guan,Q.F.;Li,H.B.;Li,H.J.;Cai,J.;Zou,Y.Acta Phys.-Chim.Sin.2012,28,1978.[林 雪,呂 鵬,關慶豐,李海波,李洪吉,蔡 杰,鄒 陽.物理化學學報,2012,28,1978.]doi:10.3866/PKU.WHXB201205172
(3) Liu,Y.F.;Ma,X.G.;Yi,X.;Zhu,Y.F.Acta Phys.-Chim.Sin.2012,28,654.[劉艷芳,馬新國,易 欣,朱永法.物理化學學報,2012,28,654.]doi:10.3866/PKU.WHXB201112232
(4) Zhang,L.;Wang,H.;Chen,Z.;Wong,P.K.;Liu,J.Appl.Catal.B:Environ.2011,106,1.
(5) Zhang,L.;Zhu,Y.Catal.Sci.Technol.2012,2,694.doi:10.1039/c2cy00411a
(6) Kudo,A.;Hijii,S.Chem.Lett.1999,10,1103.
(7) Shang,M.;Wang,W.;Sun,S.;Zhou,L.;Zhang,L.J.Phys.Chem.C 2008,112,10407.doi:10.1021/jp802115w
(8) Shang,M.;Wang,W.;Xu,H.Cryst.Growth Des.2009,9,991.doi:10.1021/cg800799a
(9) Shang,M.;Wang,W.;Ren,J.;Sun,S.;Wang,L.;Zhou,L.J.Mater.Chem.2009,19,6213.doi:10.1039/b907849e
(10)Amano,F.;Nogami,K.;Abe,R.;Ohtani,B.J.Phys.Chem.C 2008,112,9320.doi:10.1021/jp801861r
(11)Zhang,L.;Wang,W.;Chen,Z.;Zhou,L.;Xu,H.;Zhu,W.J.Mater.Chem.2007,17,2526.doi:10.1039/b616460a
(12) Shi,R.;Huang,G.;Lin,J.;Zhu,Y.J.Phys.Chem.C 2009,113,19633.doi:10.1021/jp906680e
(13) Fu,H.;Zhang,S.;Xu,T.;Zhu,Y.;Chen,J.Environ.Sci.Technol.2008,42,2085.doi:10.1021/es702495w
(14) Guo,S.;Li,X.;Wang,H.;Dong,F.;Wu,Z.J.Colloid Interface Sci.2012,369,373.doi:10.1016/j.jcis.2011.12.007
(15) Duan,F.;Zheng,Y.;Chen,M.Appl.Sci.Res.2011,257,1972.
(16)Zhou,L.;Yu,M.;Yang,J.;Wang,Y.;Yu,C.J.Phys.Chem.C 2010,114,18812.doi:10.1021/jp107061p
(17)Song,X.C.;Zheng,Y.F.;Ma,R.;Zhang,Y.Y.;Yin,H.Y.J.Hazard.Mater.2011,192,186.
(18)Zhang,L.;Man,Y.;Zhu,Y.ACS Catal.2011,1,841.doi:10.1021/cs200155z
(19)Amano,F.;Yamakata,A.;Nogami,K.;Osawa,M.;Ohtani,B.J.Phys.Chem.C 2011,115,16598.doi:10.1021/jp2051257
(20) Fu,H.;Zhang,L.;Yao,W.;Zhu,Y.Appl.Catal.B 2006,66,100.doi:10.1016/j.apcatb.2006.02.022
(21) Zhang,C.;Zhu,Y.Chem.Mater.2005,17,3537.doi:10.1021/cm0501517
(22)Amano,F.;Nogami,K.;Ohtani,B.J.Phys.Chem.C 2009,113,1536.doi:10.1021/jp808685m
(23) Chakrabarti,S.;Ganguli,D.;Chaudhuri,S.Physica E 2004,24,333.
(24)Maczka,M.;Macalik,L.;Hermanowicz,K.;KepiDski,L.;Tomaszewski,P.J.Raman Spectrosc.2010,41,1059.
(25) Graves,P.R.;Hua,G.;Myhra,S.;Thompson,J.G.J.Solid State Chem.1995,114,112.doi:10.1006/jssc.1995.1017
(26) Bordun,O.M.Inorg.Mater.1998,34,1270.

