CO2/N2 separation using supported ionic liquid membranes with green and cost-effective[Choline][Pro]/PEG200 mixtures☆
Tengteng Fan ,Wenlong Xie ,Xiaoyan Ji,Chang Liu ,Xin Feng ,*,Xiaohua Lu
1 College of Chemistry and Chemical Engineering,State Key Laboratory of Materials-Oriented Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
2 Division of Energy Science/Energy Engineering,Lule? University of Technology,97187 Lule?,Sweden
1.Introduction
Carbon dioxide(CO2)separation from the flue gas streams generated by power plant is of importance in mitigating CO2emissions.To capture CO2from the flue gas mixtures(10%–14%CO2in mostly N2),a lot of technologies have been developed including selective absorption/adsorption,catalytic conversion and membrane separation[1,2],however,the cost with the available technologies is too high for practical implementation.In general,membrane separation is an energy efficient,low-cost and environmentally friendly alternative for gas separation processes[3],and supported ionic liquid membranes(SILMs)that combine membranes and ionic liquids(ILs)for the selective separation of gases have received growing attention during recent years[4,5].The unique properties of ILs including low vapor pressure,high thermal stability and strong capillary force existing between ILs and support pores make SILMs be more competitive than conventional supported liquid membranes(SLMs)[6–8].
However,SILMs have not been industrialized because of the following reasons.Firstly,the performance of SILMs with conventional room temperature ionic liquids(RTILs)cannot meet the industrially viable standards of CO2permeability(~1000 barrer)and CO2/N2selectivity(20–40)[9,10].Scovazzo et al.[11]proposed and theoretically studied the SILMs with conventional RTILs.The results showed that the SILMs with imidazolium-based ILs only achieved a CO2permeability of 120 to 445 barrer with CO2/N2selectivity of 16 to 86[6,12].Secondly,the price of ILs is too high.SILMs based on task-specific ionic liquids(TSILs)with high CO2solubility have been proposed to increase the CO2permeability.Matsuyama et al.[13]reported amine-functionalized SILMs with the CO2permeability as high as 2500 barrer.However,these TSILs were prepared from high-cost materials with complicated synthesis route[14,15],leading a very high price for such SILMs.Generally,the price of ILs for a large scale process is 10 to 20 times higher than the conventional solvents[1].Thirdly,most of the ILs immobilized in SILMs are highly toxic and poorly biodegradable[16].For example,the inhibitory concentration(IC50)of imidazolium ILs to acetyl cholinesterase(AChE)is 82 to 990 μmol·L?1,and[bmim][PF6]was observed to be non-biodegradable[16–19].Besides,the high viscosity of ILs leads to low mass transfer rate,increasing the cost for energy and operation.This also makes ILs uncompetitive compared to the conventional solvents.
SILM scan efficiently avoid the problems caused by the high viscosity of ILs,and this has been illustrated experimentally.However,the mechanism study revealed the permeability enhancement of SILMs is very scarce.In our previous work[20,21],the kinetics of CO2absorption/desorption in IL immobilized sorbents has been studied experimentally,and the inherent mechanism for the rate enhancement was revealed based on the diffusion-reaction theory.Based on the theoretical prediction,the significant improvement of CO2flux could be obtained by tailoring the IL- film thickness.However,this theoretical prediction has not been verified experimentally in a membrane process for gas separation.
Meanwhile,choosing a proper green and cost-effective IL is also essential for the promotion of SILMs in CO2separation[22].In our previous work[23–25],to screen appropriate ILs,the theoretical energy consumption of CO2separation has been investigated,and it was found that the choline-based ILs are promising to be used as absorbents for CO2separation.Among the choline-based ILs,(2-hydroxyethyl)-trimethylammonium(S)-2-pyrrolidinecarboxylic acid salt([Choline][Pro])is classified as green and cost-effective solvent.The price,biodegradability and IC50of[Choline][Pro]are only 56 USD·kg?1,73.5%and 3500 μmol·L?1,respectively.For comparison,the price and toxicity of[Choline][Pro]are only 1/10 of those of[bmim][PF6][15,26,27].In addition,[Choline][Pro]can capture CO2effectively and be easily regenerated by blowing N2,showing a great potential to be used as a suitable IL for SILMs in CO2separation[28].
The aim of this work is to prepare green and cost-effective SILMs with good performance on CO2/N2separation and to realize the enhancement of CO2permeability by adjusting the viscosity of ILs.[Choline][Pro]with the desirable properties was chosen as the primary IL,and the addition of PEG200 was used to adjust the viscosity and to further reduce the price and toxicity.The performance on CO2/N2separation for the SILMs based on[Choline][Pro]/PEG200 mixtures was evaluated from 308.15 to 343.15 K.The effects of temperature on CO2permeability were analyzed quantitatively by Arrhenius equation.The mechanism of the effect of PEG200 on the CO2permeability was revealed based on the diffusion-reaction theory.
2.Experimental
2.1.Materials
Choline hydroxide solution(~45 wt%in methanol)was purchased from Sigma Aldrich Chemical.L-proline(98%)was produced by Chinese Huixing Medicine Corporation Ltd.PEG200(analytical grade)with average molecular weight of 200 g·mol?1was produced by Guangdong Sci-Tech Corporation Ltd.The hydrophilic polyethersulfone(PES)membrane was purchased from Beijing Membrane Corporation with the specifications of(pore size:0.22 μm;porosity:80%;thickness:120 μm;and effective cross-sectional area:51.5 cm2).Methanol(99.5%)was supplied by Shanghai Lingfeng Chemical Reagent Co.Ltd.KBr(FT-IR grade)was purchased from Sigma-Aldrich Chemical.N2and CO2with a purity of 99.99%(mole fraction)were obtained from Nanjing Gas Supply Inc.,China.All the chemicals and materials were used as received.
2.2.Synthesis of IL
The procedure to synthesize[Choline][Pro]was similar to that described by the research group of Han[29].Shortly,the IL was synthesized simply by neutralization of choline hydroxide and L-proline.The reaction last for 48 h at room temperature.Subsequently,an appropriate amount of ethyl acetate was added to remove unreacted L-proline.The filtrate was evaporated to remove solvents.All the IL samples and PEG200 were dried under vacuum at 353.15 K for at least 48 h before use.The structures of[Choline][Pro]are shown in Fig.1.
2.3.SILMs preparation
The[Choline][Pro]/PEG200 mixtures were prepared using an analytical balance with an uncertainty of±10?4g.Thoroughly mixing was assured by magnetic stirring for 4 h in closed vials.In this work,the mass ratios of[Choline][Pro]/PEG200 mixtures were 1:0,1:1,1:2(i.e.W[Choline][Pro]:WPEG200=1:0,1:1,1:2).The prepared mixtures were dried under vacuum at 353.15 K for 48 h.
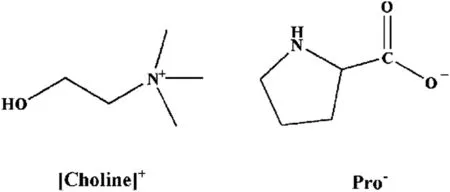
Fig.1.Chemical structures of[Choline][Pro].
The SILMs were prepared using the impregnation method.Brie fly,the SILMs were formed by soaking the PES-membrane into[Choline][Pro]/PEG200 mixtures in a vacuum dry oven at 313.15 K for 24 h to completely saturate the membrane.The excess liquids on the membrane surface were then wiped away with dustless paper prior to the membrane installation into a test unit.In addition,the leakage of SILMs was tested with N2before the gas permeation experiments.
2.4.Characterization
2.4.1.Fourier transform infrared(FTIR)
IR spectra of the[Choline][Pro]were recorded on a FTIR spectrometer(Bruker TENSOR 27,Germany)with KBr.
2.4.2.1H nuclear magnetic resonance(NMR)
1H NMR spectra of[Choline][Pro]were recorded in D2O at 300 MHz by a NMR spectrometer at 300 MHz(Bruker AVANCE Digital 300 MHz NMR,Germany)to confirm the structure of the synthesized ILs.
2.4.3.Thermogravimetric analysis(TGA)
The thermal properties of the[Choline][Pro]/PEG200 mixtures and the supported membranes were detected by thermogravimetric analysis(TGA).TGA analyses were performed using a Netzsch TG209 F3 thermal analyzer in a N2atmosphere with a flow rate of40 ml·min?1.About 10 mg samples were placed in an Al2O3sample cell.The scan was carried out at a heating rate of 10 K·min?1at temperatures from the room temperature to 1000 K.
2.4.4.Field emission scanning electron microscopy(FESEM)
The morphologies of SILMs were evaluated by FESEM system(Hitachi S-4800,FEI,Japan)at the room temperature.The surface of the membranes was sputtered with gold and then observed.
2.5.Gas permeation experiment
Gas permeation properties were studied using a stainless steel membrane permeation cell with an effective membrane area of 51.5 cm2.The experimental apparatus is shown in Fig.2[30].In a permeation experiment,moisture in the feed gases was removed with a molecular sieve trap prior to contacting the SILMs.The feed gas pressure was adjusted with a pressure gauge while the pressure of permeate side was atmosphere.A trans membrane pressure difference was set to be 0.18 MPa.The flow rate of the permeate gas was measured with a bubble flow meter.The temperature was maintained at 308.15,313.15,323.15,333.15 and 343.15 K,respectively,using a temperature control system.All measurements were performed in triplicate,and the average values were reported and used for further analysis.The permeability was calculated based on the effective area and the thickness of PES membrane.
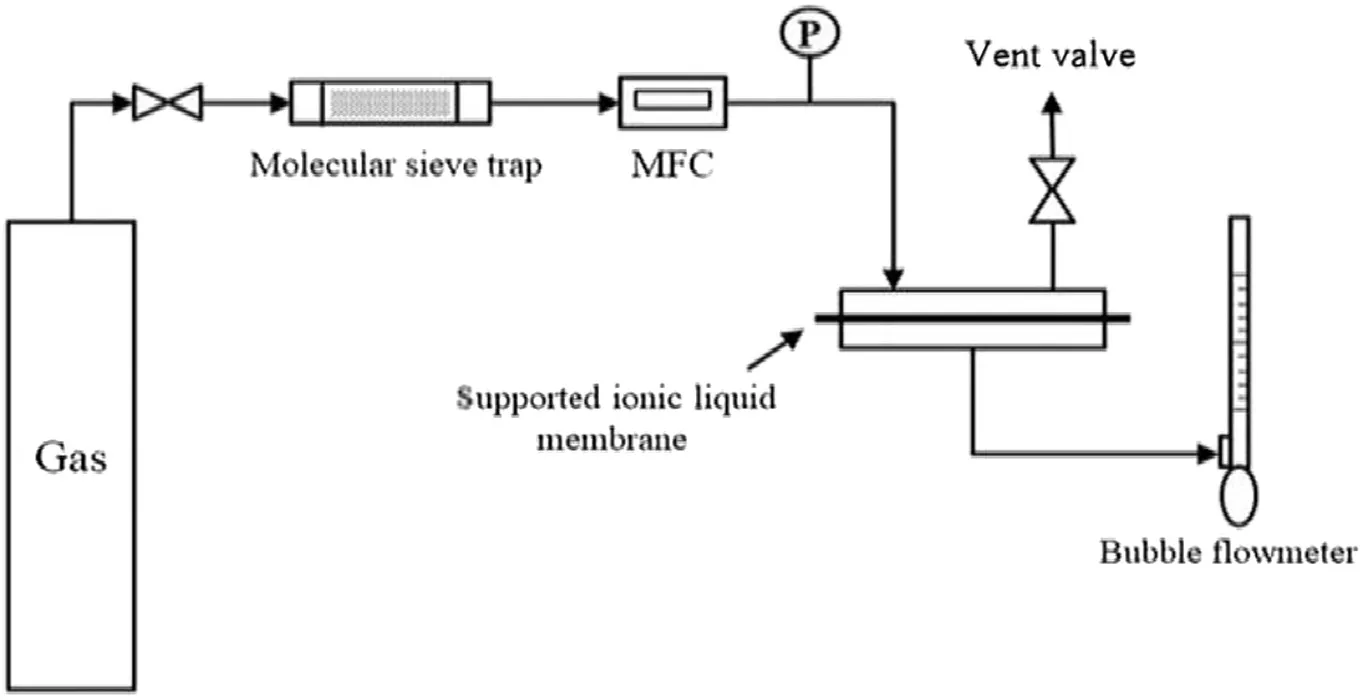
Fig.2.Gas permeation apparatus.
The permeability of gas i through SIL Msin barrercan be calculated as Eq.(1),
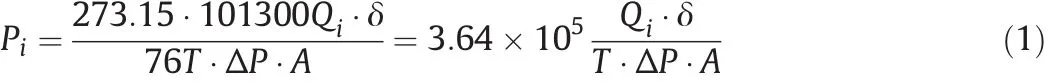
where Piis the permeability of gas i(i represents CO2or N2)in barrer(=10?10cm3(STP)·cm·(cm2·s·cmHg)?1),Qiis the flow rate ofpermeate gas i in cm3·s?1,δ is the membrane thickness in cm,T is the temperature in K,Δp is the cross-membrane pressure difference in MPa,and A is the effective area of membrane,51.5 cm2.
The ideal selectivity for a pair of gases(i,j)can be calculated as Eq.(2),

3.Results and Discussion
3.1.Characterization
3.1.1.Structure of[Choline][Pro]
IR:3406.5,2971.6,2879.8,1582.0,1479.2,1398.3,1348.4,1087.2,1055.2,955.7 cm?1.The results are in good agreement with the values reported in the literature[15].The structure of the[Choline][Pro]was confirmed by1H NMR,δ:1.46–1.52(m,3H,CH2,CH2),1.86–1.89(m,1H,CH2),2.48–2.52(m,1H,CH2-N),2.80–2.83(m,1H,CH2-N),2.98(s,9H,CH3,CH3,CH3),3.18–3.23(m,1H,CH-N),3.27–3.31(t,2H,CH2),3.79–3.84(m,2H,CH2).
3.1.2.Densities and viscosities of[Choline][Pro]/PEG200
The densities and viscosities of[Choline][Pro]/PEG200 mixtures were measured in the atmospheric pressure at temperatures from 308.15 to 333.15 K,and the results are shown in Figs.3 and 4.Both the density and viscosity decreased with the addition of PEG200,i.e.the density and viscosity of[Choline][Pro]/PEG200 follow the trend of W[Choline][Pro]:W PEG200=1:0>W[Choline][Pro]:W PEG200=1:1>
W[Choline][Pro]:WPEG200=1:2.It was also observed that the density and viscosity of[Choline][Pro]/PEG200 mixtures decreased with increasing temperature.These results were similar to those for other amino acid-based ILs[31,32].
3.1.3.Thermal properties
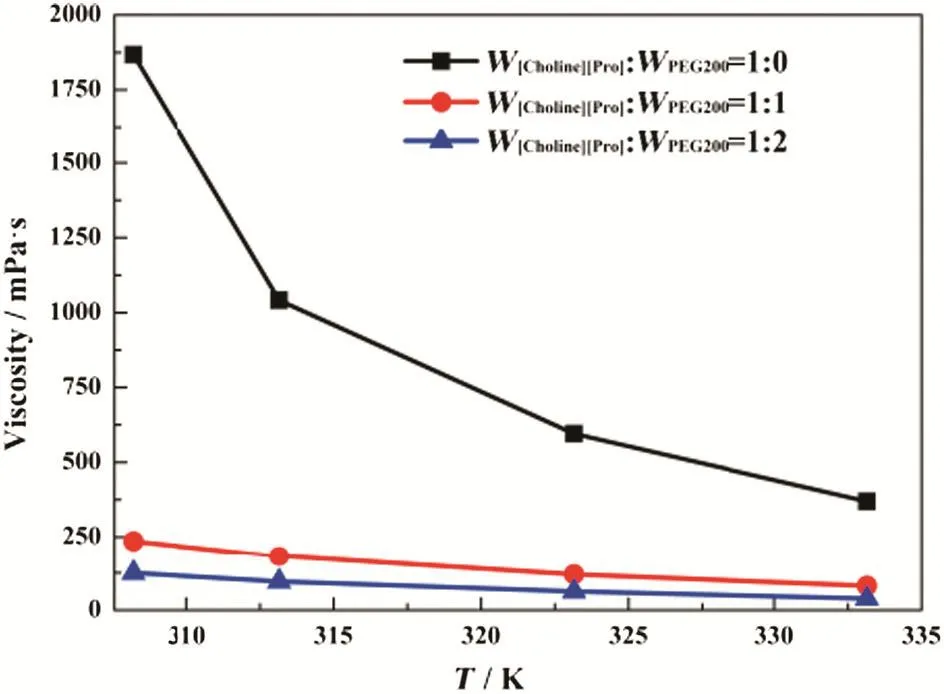
Fig.3.Effect of temperature on the viscosity of[Choline][Pro]/PEG200 mixtures.
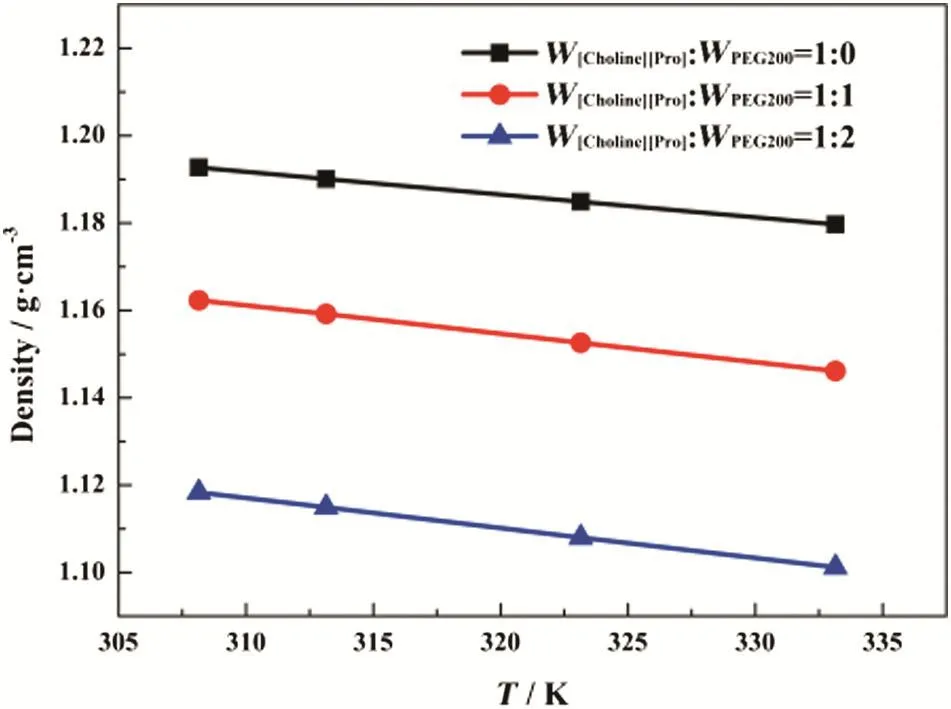
Fig.4.Effect of temperature on the density of[Choline][Pro]/PEG200 mixtures.
TGA was used to investigate the effect of PEG200 content on the thermal properties of the liquids and the membranes(i.e.the onset temperature(Tonset)).According to the TGA curves shown in Fig.5,we can conclude that the[Choline][Pro]/PEG200 mixtures and membranes are thermally stable up to 440 K under N2atmosphere.The Tonsetdetected in this work is listed in Table 1 together with other properties,such as molecular weight,viscosity and density.With the addition of PEG200,the Tonsetbecame higher.These results suggest that all the liquids and membranes are thermally stable up to high temperatures,and this is an essential feature for industrial applications.
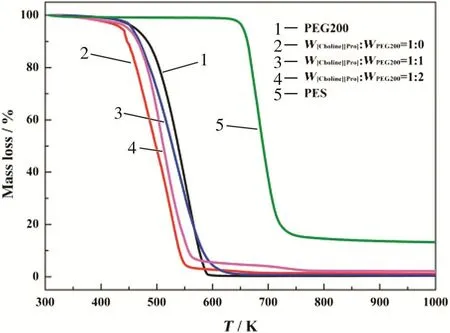
Fig.5.TGA analyses for PEG200,[Choline][Pro]/PEG200 mixtures,and PES.
3.1.4.Surface morphology of SILMs
SEM images were detected to study the surface morphology of the prepared membranes.As it can be seen from Fig.6,the liquids were homogeneously distributed,almost completely filling the pores of the membranes,and only the largest pores were partially filled.These images showed the presence of excess liquids on the membrane surface,especially for the case of the membrane immobilized with pure[Choline][Pro](Fig.6b).With the addition of PEG200,less excess of liquids were observed as shown in Fig.6c and 6d.
3.2.Performance on CO2/N2 separation
3.2.1.Permeability(P)
The permeability of pure CO2and N2was studied at temperatures from 308.15 to 343.15 K,and the experimental results are listed in Table 2.Based on the measured permeability for the pure gas,the ideal selectivity of CO2/N2was estimated with Eq.(2).As shown in Table 2,the CO2permeance for these SILMs is in the range of 343.3–1798.6 barrer and the CO2/N2selectivity is between 7.9 and 34.8.Particularly,when the mass ratio of[Choline][Pro]to PEG200 is 1:1,the permeance and selectivity meet the industrially viable standards with high CO2permeability(~1000 barrer)and good CO2/N2selectivity(20–40)[9,10].
Based on the experimental measurements,it was observed that the gas permeability for both CO2and N2increased with increasing temperature.The Arrhenius equation as described in Eq.(3)was used to describe the temperature effect on the gas permeability.

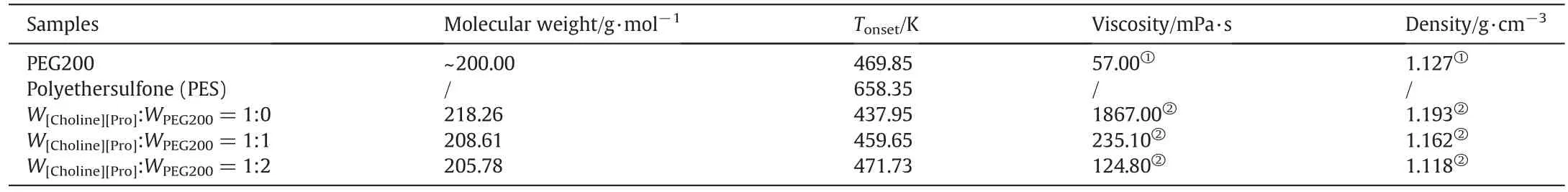
Table 1 The molecular weight,T onset,viscosity and density of the studied samples.
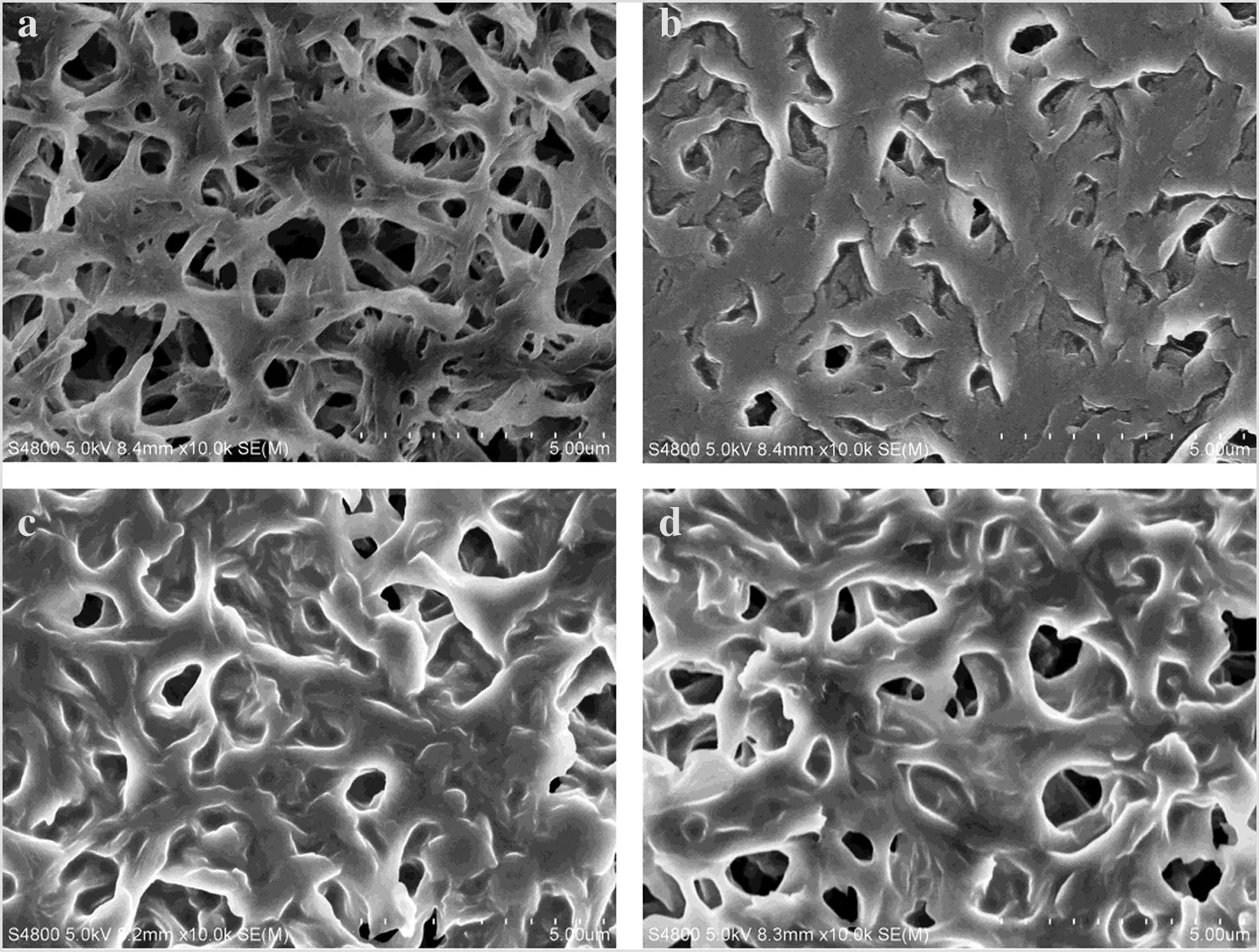
Fig.6.SEM images of surface morphology of the prepared membranes.(a)PES membrane;(b),(c)and(d):SILMs of[Choline][Pro]/PEG200 mixtures with different mass ratios of[Choline][Pro]to PEG200.(b)1:0;(c)1:1;and(d)1:2.

Table 2 Gas permeability and selectivity for CO2 and N2 through the synthesized SILMs at temperatures from 308.15 to 343.15 K.
where P0is the pre-exponential factor and EPis the activation energy of permeation.
The modeling results are illustrated in Fig.7.As ln P shows a linearrelationship with 1/T,the Arrhenius equation with a constant EPcan be used to describe the effect of the temperature on the gas permeability,and EPcan be calculated from the slopes of the linear curve.The values of EPfor CO2and N2at different compositions are listed in Table 3.
The idealselectivity ofCO2/N2is depicted in Fig.8.The idealselectivity decreased with increasing temperature.This observation agrees well with the activation energies estimated in this work.As shown in Table 3,the activation energies of N2permeation are higher than those of CO2,which means that the permeability of N2is more sensitive to the temperature.The temperature increase will enhance more on N2permeation than that of CO2,leading a lower CO2/N2selectivity.Therefore,we can conclude that a low temperature has a positive in fluence on the CO2/N2selectivity.
3.2.2.Solubility(S)
The solubility of CO2in[Choline][Pro]/PEG200 mixtures at 308.15,323.15,338.15 and 353.15 K has been measured by the research group of Han[28].Based on their experimental results,the solubility of CO2at 313.15,333.15 and 343.15 K were estimated with inter-or extrapolation and the solubility data were listed in Table S1(available as supplementary material).
The effect of temperature on the CO2solubility can be well described by the Arrhenius equation(Eq.(4)):

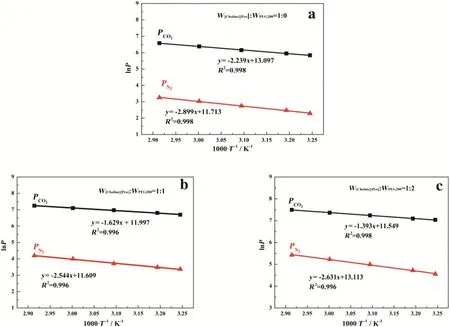
Fig.7.ln P of CO2 and N2 through SILMs plotted against 1000 T?1 at W[Choline][Pro]:W PEG200=1:0(a),1:1(b)and 1:2(c).Solid lines are the linear regression of the data.Symbols are experimental data.

Table 3 Experimental activation energies of permeation(E P),partial molar enthalpy(ΔH s)and activation energy of diffusion(E D)of SILMs based on[Choline][Pro]/PEG200 mixtures.
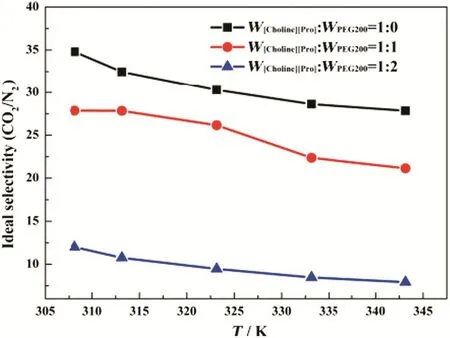
Fig.8.Effect of temperature on the selectivity of CO2/N2 through the SILMs.
where S0is the pre-exponential factor,and ΔHsis the partial molar enthalpy of absorption.
The modeling results are illustrated Fig.9 with good agreement with experimental data.As ln S follows a linear relationship with 1/T,ΔHscan be calculated from the slopes of the linear curve.The values of ΔHsat different compositions are listed in Table 3.The negative ΔHsvalues indicate that heat is released during the CO2absorption process and the interaction between IL and CO2is strong.The addition of PEG200 will only slightly change the partial molar enthalpy ΔHsbased on the values listed in Table 3.
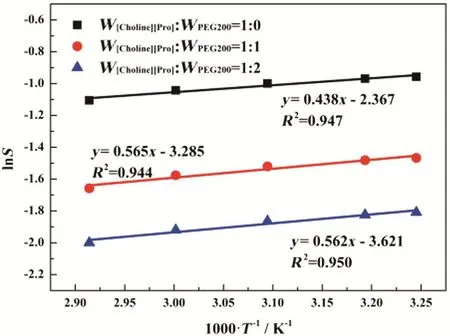
Fig.9.Dependence of ln S on 1000 T?1 at W[Choline][Pro]:W PEG200=1:0,1:1 and 1:2.Solid lines are the linear regression of the data,and symbols are the gas solubility data.
3.2.3.Diffusivity(D)
Based on the gas permeability(P)and the gas solubility in the IL(S),the diffusion coefficients(D)can be calculated via solution–diffusion mechanism according to Eq.(5):

In this work,the CO2diffusion coefficient D in[Choline][Pro]/PEG200 was calculated from its permeability measured in this work and the corresponding solubility(Section 3.2.2)and the results were listed in Table S2(available as supplementary material).The temperature effect on the gas diffusivity was further described by Eq.(6)and shown in Fig.10.ln D follows a linear relationship with 1/T,and EDcan be calculated from the slopes of the linear curve.The values of EDat different compositions are listed in Table 3.
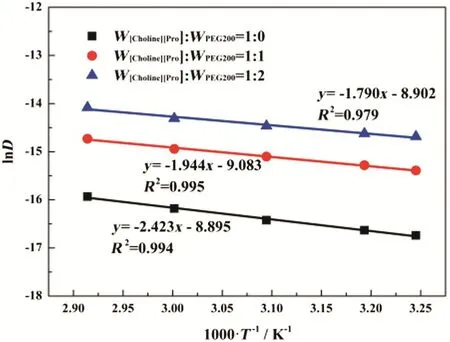
Fig.10.Dependence of ln D on 1000 T?1 at W[Choline][Pro]:W PEG200=1:0,1:1 and 1:2.Solid lines are the linear regression of the data.Symbols are the estimated gas diffusivity.

where D0is the pre-exponential factor,and EDis the activation energy of diffusion.
Based on the results listed in Table 3,for CO2,we can observe that the estimated activation energy for the permeability(EP)agree with the algebraic summation of the activation energy for the diffusivity(ED)and the partial molar enthalpy of absorption(ΔHs).Therefore,we can conclude that the influences of the temperature on the permeability,solubility and diffusivity were successfully described by Arrhenius type equation.
3.3.Effect of PEG200 on CO2 permeability
3.3.1.Permeability and viscosity
The high viscosity of ILs is a well-known challenge to the diffusivity of gases in permeation[33].To investigate how the viscosity affects the permeability,the permeability of CO2was correlated to the viscosity of[Choline][Pro]/PEG200 mixtures at temperatures from 308.15 to 333.15 K.As shown in Fig.11,the CO2permeability increases with decreasing viscosity of[Choline][Pro]/PEG200 mixtures.Furthermore,when the viscosity is high,the decrease of the viscosity will increase the CO2permeability,but it is not significant.However,when the viscosity is low,even a slightly decrease of the viscosity will lead to a remarkable increase of the CO2permeability.Forexample,the CO2permeability increases only~250 barrer when the viscosity decreases from 1900 to 370 mPa·s;when the viscosity decreases from 240 to 38 mPa·s,the CO2permeability increases~800 barrer.
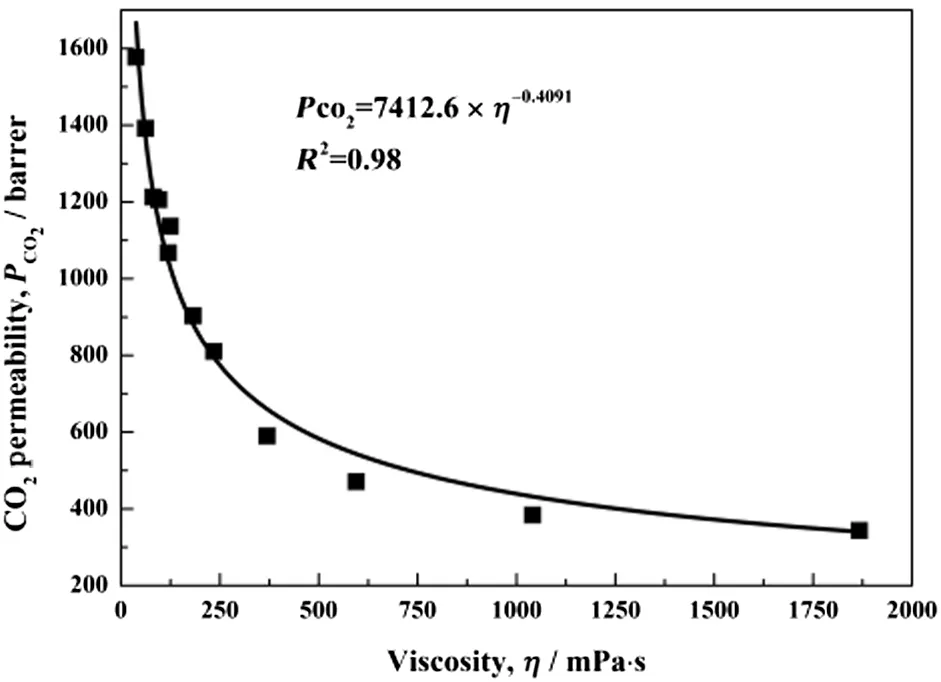
Fig.11.CO2 permeability through SILMs as a function ofviscosity of[Choline][Pro]/PEG200 mixtures with mass ratios of 1:0,1:1 and 1:2.
The relationship between the CO2permeability and the viscosity of[Choline][Pro]/PEG200 mixtures can be represented by Eq.(7):

where PCO2and η are the CO2permeability in SILMs and the viscosity of[Choline][Pro]/PEG200 mixtures,respectively.
This empirical correlation combined a wide range of the viscosities of[Choline][Pro]/PEG200 mixtures with CO2permeability,and it can be used to predict the CO2permeability for this series of mixtures.Based on this empirical correlation equation,the estimation shows that the SILMs with high CO2permeability(~1000 barrer)can be achieved by tailoring the viscosity down to 120 mPa·s.
3.3.2.Mechanism study
The effect of PEG200 on the CO2permeability of the prepared SILMs was investigated experimentally in this work,and the addition of PEG200 led to a high CO2permeability.Forexample,the CO2permeability in[Choline][Pro]/PEG200 mixture can be 3 times higher than that of pure[Choline][Pro]at 308.15 K.To reveal the mechanism behind this experimental observation,the CO2permeability in the SILMs at 308.15 K was chosen as an example for the further analysis.
Following our previous work[20,21,34,35],the linear nonequilibrium thermodynamics was used to study the CO2permeability with the chemical potential gradient(ΔμCO2)as the driving force:

whereis the apparent chemical-potential-based mass-transfer coefficient,are the chemical potentials of CO2in the IL at feed and permeate sides,respectively,and ΔμCO2is the chemical potential gradient.
The chemical potential of CO2in IL can be calculated according to.
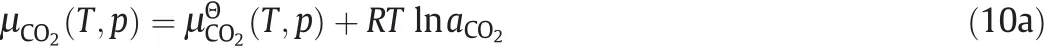
where(T,p)is the standard chemical potential,p is the partial pressure of CO2,and aCO2is the activity of CO2in IL.
In this work,we assumed that the non-ideal behavior ofCO2in the IL can be neglected due to the low CO2solubility[36],which means that the activity coefficient of CO2in the IL equals to one,and the activity of CO2in the IL equals to the corresponding concentration,i.e.γCO2=1,and aCO2=xCO2.Therefore,we have.

According to our previous work[20,21],the CO2absorption/desorption process in IL was assumed to comprise two steps:surface-reaction and diffusion.In the surface-reaction layer,IL in sorbents contacts CO2in the vapor phase,CO2chemically or physically dissolves into IL.In the diffusion layer,the dissolved CO2is transported away from the interface through the liquids.Neglecting the resistances from the solid support and the permeate side by using the effective membrane area,the overall resistance(1/Kμ)of the process can be considered as the summation of the resistances from the reaction layer and the diffusion layer as described in Eq.(12),

where Kμ,sis the surface-reaction chemical-potential-based masstransfer coefficient and Kμ,dis the diffusion chemical-potential-based mass-transfer coefficient.The resistance from the reaction layer(1/Kμ,s)is due to the chemical contribution of[Choline][Pro]and depends on the temperature[20].The diffusion chemical-potential-based mass trans fer coefficient Kμ,dis related to the diffusivity and the thickness of diffusion layer(d),that is,the SILMs thickness,

The effect of the viscosity(η)on the overall resistance(1/Kμ)for the CO2separation in SILMs at 308.15 K is illustrated in Fig.12,which shows that the overall resistance(1/Kμ)decreases with decreasing the viscosity of ILs.As mentioned,the resistance from the reaction layer is a constant at the same temperature.1/Kμ,swas then obtained based on the results of Fig.12 with the value of 2367.46,that is,1/Kμ,s=2367.46.
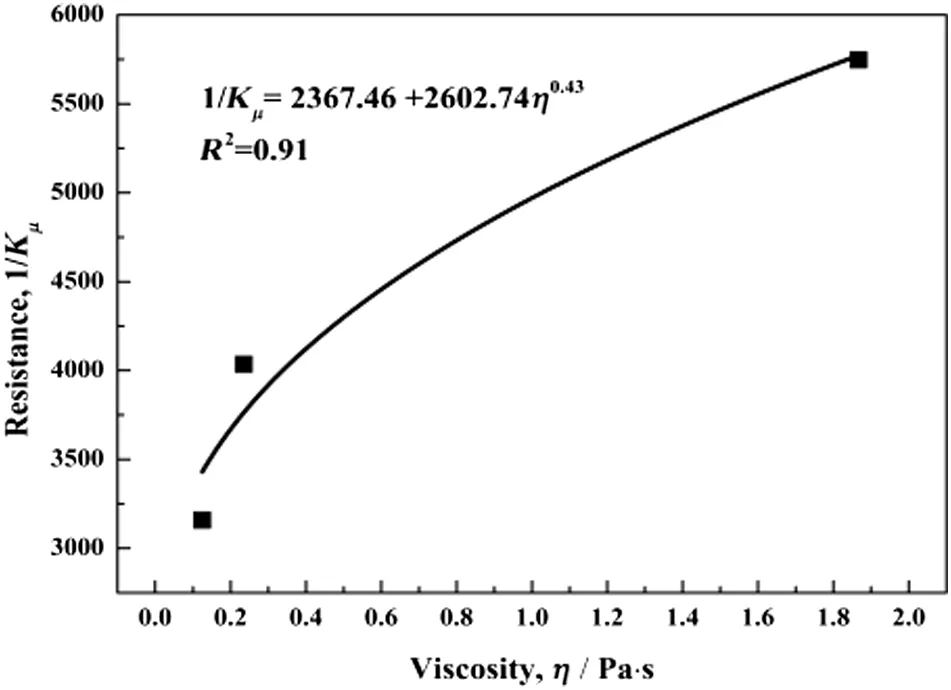
Fig.12.Resistance of CO2 mass transfer in SILMs with different viscosities at 308.15 K.
The resistance from the diffusion layer with different PEG200 content can be obtained by applying Eq.(12)and 1/Kμ,s=2367.46 as listed in Table 4.It can be seen that the process was altered from the diffusion control to surface-reaction control,and the addition of PEG200 resulted in a decrease of the overall resistance due to the decrease of the resistance from the diffusion layer.For example,when the SILM was immobilized by pure[Choline][Pro],the overall resistance is 5746.4,and the resistance from the diffusion layer(1/Kμ,d)contributes 58.8%to the overall;while the diffusion layer contributes only 25%(2 times lower)when the[Choline][Pro]/PEG200 mass ratio is 1:2,and the overall resistance decreases down to 3157.5.This further explains the CO2permeability enhancement for the process with a viscosity reducer(PEG200)and points out a direction for designing membranes with high gas permeability.

Table 4 Resistances for the CO2 mass transfer in SILMs at 308.15 K.
3.4.Comparison with other SILMs
The trade-off relationship between permeability and selectivity for gas separation with membrane is presented in a Robeson plot[39].Comparing the results reported in this work to the upper-bound values of the traditional polymer membranes and to the results of others SILMs[37,40,41],it can be concluded that most of the SILMs have better performance than the traditional polymer membranes studied previously as shown in Fig.13.The performances of PES membrane impregnated with[Choline][Pro]/PEG200 mixtures are better than those with ammonium-RTILs reported by the Scovazzo group[40].Although,the ideal selectivity values are smaller than those imidazolium-based SILMs,the CO2permeability is as good as them[41].When it comes to the price,toxicity and biodegradability,as listed in Table 5,SILMs based on[Choline][Pro]/PEG200 mixtures are much competitive than SILMs with imidazolium-based RTILs[15,26].Therefore,SILMs with[Choline][Pro]/PEG200 mixtures have the potential to be used ata larger scale.
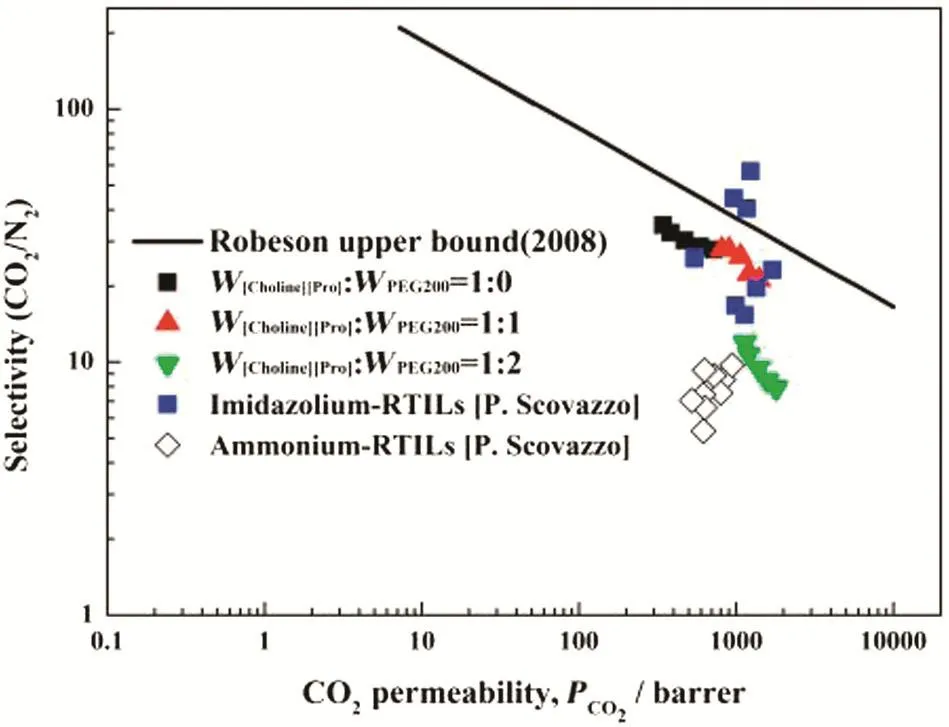
Fig.13.Comparison of SILMs with imidazolium-RTILs,ammonium-RTILs and[Choline][Pro]/PEG200 mixtures with Robeson upper bound.
4.Conclusions
We proposed green and cost-effective SILMs with[Choline][Pro]/PEG200 mixturesfor CO2separation from flue gases.The CO2/N2separation performance of these SILMs met industrially viable standards with CO2permeability from 343.3 to 1798.6 barrer and CO2/N2selectivity from 7.9 to 34.8.The comparison with other SILMs shows that SILMs with[Choline][Pro]/PEG200 mixtures are much more competitive than SILMs with imidazolium-based RTILs.The effects of temperature on permeability,solubility and diffusivity were studied and described quantitatively by Arrhenius equation.In addition,the dependence of the CO2permeability on the IL viscosity indicates that CO2permeability increases dramatically by decreasing the viscosity when the viscosity is lower than 370 mPa·s.The analysis with the diffusion-reaction theory revealed the inherent mechanism for the permeability enhancement,that is,with the addition of PEG200,the SILMs process was altered from diffusion-control to reaction-control.This work will benefit the future study on the application of environmentally benign and economically cost-effective SILMs for CO2separation.
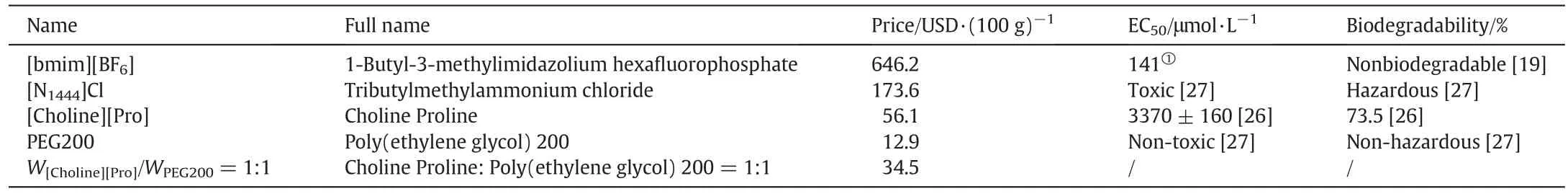
Table 5 The price,toxicity and biodegradability for traditional ILs and[Choline][Pro]/PEG200.

Supplementary Material
Supplementary data to this article can be found on line at http://dx.doi.org/10.1016/j.cjche.2016.03.006.
[1]M.Ramdin,T.W.De Loos,T.J.H.Vlugt,State-of-the-art of CO2capture with ionic liquids,Ind.Eng.Chem.Res.51(24)(2012)8149–8177.
[2]K.Huang,X.M.Zhang,Y.X.Li,Y.T.Wu,X.B.Hu,Facilitated separation of CO2and SO2through supported liquid membranes using car boxy late-based ionic liquids,J.Membr.Sci.471(6)(2014)227–236.
[3]Y.Peng,Y.Li,Y.Ban,H.Jin,W.Jiao,X.Liu,W.Yang,Metal–organic framework nanosheets as building blocks for molecular sieving membranes,Science 346(6215)(2014)1356–1359.
[4]M.A.Malik,M.A.Hashim,F.Nabi,Ionic liquids in supported liquid membrane technology,Chem.Eng.J.171(1)(2011)242–254.
[5]P.Uchytil,J.Schauer,R.Petrychkovych,K.Setnickova,S.Y.Suen,Ionic liquid membranes for carbon dioxide-methane separation,J.Membr.Sci.383(s 1–2)(2011)262–271.
[6]J.E.Bara,T.K.Carlisle,C.J.Gabriel,D.Camper,A.Finotello,D.L.Gin,R.D.Noble,Guide to CO2separations in imidazolium-based room-temperature ionic liquids,Ind.Eng.Chem.Res.48(6)(2009)2739–2751.
[7]L.A.Blanchard,D.Hancu,E.J.Beckman,J.F.Brennecke,Green processing using ionic liquids and CO2,Nature 399(6731)(1999)28–29.
[8]S.H.Barghi,M.Adibi,D.Rashtchian,An experimental study on permeability,diffusivity,and selectivity of CO2and CH4through[bmim][PF6]ionic liquid supported on an alumina membrane:Investigation of temperature fluctuations effects,J.Membr.Sci.362(1)(2010)346–352.
[9]P.T.Nguyen,B.A.Voss,E.F.Wiesenauer,D.L.Gin,R.D.Noble,Physically gelled roomtemperature ionic liquid-based composite membranes for CO2/N2separation:Effect of composition and thickness on membrane properties and performance,Ind.Eng.Chem.Res.52(26)(2013)8812–8821.
[10]T.C.Merkel,H.Lin,X.Wei,R.Baker,Power plant post-combustion carbon dioxide capture:An opportunity for membranes,J.Membr.Sci.359(s 1–2)(2010)126–139.
[11]P.Scovazzo,A.E.Visser,J.H.Davis,R.D.Rogers,C.A.Koval,D.L.DuBois,R.D.Noble,Supported ionic liquid membranes and facilitated ionic liquid membranes,Chem In form Wiley 2002,pp.69–87.
[12]P.Jindaratsamee,Y.Shimoyama,H.Morizaki,A.Ito,Effects of temperature and anion species on CO2permeability and CO2/N2separation coefficient through ionic liquid membranes,J.Chem.Thermodyn.43(3)(2011)311–314.
[13]S.Hanioka,T.Maruyama,T.Sotani,M.Teramoto,H.Matsuyama,K.Nakashima,M.Hanaki,F.Kubota,M.Goto,CO2separation facilitated by task-specific ionic liquids using a supported liquid membrane,J.Membr.Sci.314(s 1–2)(2008)1–4.
[14]E.D.Bates,R.D.Mayton,N.Ioanna,J.H.Davis,CO2capture by a task-specific ionic liquid,J.Am.Chem.Soc.124(6)(2002)926–927.
[15]Q.P.Liu,X.D.Hou,N.Li,M.H.Zong,Ionic liquids from renewable biomaterials:Synthesis,characterization and application in the pretreatment of biomass,Green Chem.14(2)(2012)304–307.
[16]M.Petkovic,K.R.Seddon,L.P.Rebelo,C.Silva Pereira,Ionic liquids:A pathway to environmental acceptability,Chem.Soc.Rev.40(27)(2011)1383–1403.
[17]M.T.Garcia,N.Gathergood,P.J.Scammells,Biodegradable ionic liquids:Part II.Effect of the anion and toxicology,Green Chem.1(2005)9–14.
[18]J.Arning,S.Stolte,A.B?schen,F.Stock,W.R.Pitner,U.Welz-Biermann,B.Jastorff,J.Ranke,Qualitative and quantitative structure activity relationships for the inhibitory effects of cationic head groups,functionalised side chains and anions of ionic liquids on acetylcholinesterase,Green Chem.10(1)(2008)47–58.
[19]D.Zhao,Y.Liao,Z.Zhang,Toxicity of ionic liquids,Clean–Soil Air Water 35(1)(2007)42–48.
[20]W.Xie,X.Ji,X.Feng,X.Lu,Mass-transfer rate enhancement for CO2separation by ionic liquids:Theoretical study on the mechanism,AIChE J.61(12)(2015)4437–4444.
[21]X.Lu,Y.Ji,X.Feng,X.Ji,Non-equilibrium thermodynamics analysis and its application in inter facial mass transfer,Sci.China Chem.41(10)(2011)1540–1547.
[22]E.Santos,J.Albo,A.Irabien,Acetate based supported ionic liquid membranes(SILMs)for CO2separation:Influence of the temperature,J.Membr.Sci.452(4)(2014)277–283.
[23]Y.Zhang,X.Ji,Y.Xie,X.Lu,Screening of conventional ionic liquids for carbon dioxide capture and separation,Appl.Energy 162(2016)1160–1170.
[24]Y.Zhang,X.Ji,X.Lu,Energy consumption analysis for CO2separation from gas mixtures,Appl.Energy 130(5)(2014)237–243.
[25]Y.Zhang,X.Ji,X.Lu,Properties and applications of choline chloride/urea and choline chloride/glycerol,Sci.China Chem.44(6)(2014)927–941.
[26]X.D.Hou,Q.P.Liu,T.J.Smith,N.Li,M.H.Zong,Evaluation of toxicity and biodegradability of cholinium amino acids ionic liquids,PLoS One 8(3)(2013)e59145.
[27]Sigma-Aldrich,http://www.sigmaaldrich.com/china-mainland.html.
[28]X.Li,M.Hou,Z.Zhang,B.Han,G.Yang,X.Wang,L.Zou,Absorption of CO2by ionic liquid/polyethylene glycol mixture and the thermodynamic parameters,Green Chem.10(8)(2008)879–884.
[29]S.Hu,T.Jiang,Z.Zhang,A.Zhu,B.Han,J.Song,Y.Xie,W.Li,Functional ionic liquid from biorenewable materials:Synthesis and application as a catalyst in direct aldol reactions,Tetrahedron Lett.48(2007)5613–5617.
[30]F.W.Wu,L.Li,Z.H.Xu,S.J.Tan,Z.B.Zhang,Transport study of pure and mixed gases through PDMS membrane,Chem.Eng.J.117(1)(2006)51–59.
[31]D.J.Tao,Z.Cheng,F.F.Chen,Z.M.Li,N.Hu,X.S.Chen,Synthesis and thermophysical properties of biocompatible cholinium-based amino acid ionic liquids,J.Chem.Eng.Data 58(6)(2013)1542–1548.
[32]S.Kasahara,E.Kamio,H.Matsuyama,Improvements in the CO2permeation selectivities of amino acid ionic liquid-based facilitated transport membranes by controlling their gas absorption properties,J.Membr.Sci.454(6)(2014)155–162.
[33]N.Shahkaramipour,M.Adibi,A.A.Seifkordi,Y.Fazli,Separation of CO2/CH4through alumina-supported geminal ionic liquid membranes,J.Membr.Sci.455(4)(2014)229–235.
[34]Y.Demirel,S.I.Sandler,Nonequilibrium thermodynamics in engineering and science,J.Phys.Chem.B 108(1)(2003)31–43.
[35]X.Lu,Y.Ji,X.Feng,X.Ji,Methodology of non-equilibrium thermodynamics for kinetics research of CO2capture by ionic liquids,Sci.China Chem.55(6)(2012)1079–1091.
[36]H.Liu,H.Tian,H.Yao,D.Yu,W.Zhao,X.Bai,Improving physical absorption of carbon dioxide by ionic liquid dispersion,Chem.Eng.Technol.36(8)(2013)1402–1410.
[37]D.Morgan,L.Ferguson,P.Scovazzo,Diffusivities of gases in room-temperature ionic liquids:data and correlations obtained using a lag-time technique,Ind.Eng.Chem.Res.44(13)(2005)4815–4823.
[38]L.Ferguson,P.Scovazzo,Solubility,diffusivity,and permeability of gases in phosphonium-based room temperature ionic liquids:Data and correlations,Ind.Eng.Chem.Res.46(4)(2007)1369–1374.
[39]L.M.Robeson,The upper bound revisited,J.Membr.Sci.320(s 1–2)(2008)390–400.
[40]R.Condemarin,P.Scovazzo,Gas permeabilities,solubilities,diffusivities,and diffusivity correlations for ammonium-based room temperature ionic liquids with comparison to imidazolium and phosphonium RTIL data,Chem.Eng.J.147(1)(2009)51–57.
[41]P.Scovazzo,Determination of the upper limits,benchmarks,and critical properties for gas separations using stabilized room temperature ionic liquid membranes(SILMs)for the purpose of guiding future research,J.Membr.Sci.343(s 1–2)(2009)199–211.
 Chinese Journal of Chemical Engineering2016年11期
Chinese Journal of Chemical Engineering2016年11期
- Chinese Journal of Chemical Engineering的其它文章
- COSMO-RS:An ionic liquid prescreening tool for gas hydrate mitigation☆
- Synthesis,characteristics of hierarchical EU-1 zeolite for xylene isomerization probe reaction☆
- Design and control of methyl acetate-methanol separation via heat-integrated pressure-swing distillation
- Solving chemical dynamic optimization problems with ranking-based differential evolution algorithms☆
- An analysis of an ethanol-based,whole-crop refinery system in China☆
- Simultaneous hybrid modeling of a nosiheptide fermentation process using particle swarm optimization☆
