Effects of porous oxide layer on performance of Pd-based monolithic catalysts for 2-ethylanthraquinone hydrogenation☆
Xin Shi,Enxian Yuan ,Guozhu Liu ,2,Li Wang ,2,*
1 Key Laboratory for Green Chemical Technology of the Ministry of Education,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
2 Collaborative Innovation Center of Chemical Science and Engineering(Tianjin),Tianjin 300072,China
1.Introduction
Hydrogen peroxide(H2O2)has a wide range of industrial uses as a green oxidizer,such as chemical synthesis,water treatment,food processing,medical sterilization,pulp bleach,and propellant[1–3].At present,H2O2is produced almost exclusively by the anthraquinone auto-oxidation(AO)process.In the AO process(as shown in Fig.1),a mixture of anthraquinone derivatives (AQs,commonly,2-ethylanthraquinone and 5,6,7,8-tetrahydro-2-ethylanthraquinone)dissolved in a mixture of organic solvents is first hydrogenated in the presence of a catalyst(commonly,Pd-based catalyst)to their corresponding hydroquinone.Then,formed hydroquinone is oxidized by molecular oxygen to produce hydrogen peroxide with regenerating AQs.H2O2is then extracted with water to obtain an aqueous solution[4–6].Among the main steps mentioned above,the liquid-phase hydrogenation of AQs is the key one affecting the yield of H2O2and consumption of raw materials[7].In fact,some secondary reactions(generally deep hydrogenation reactions,such as aromatic ring hydrogenation and C--O bond hydrogenolysis of hydroquinone)also occur,which lead to the loss of AQs and hydrogen.Drelinkiewicz et al.point out that all the side reactions can be considered as consecutive to hydrogenation of 2-ethylanthraquinone(eAQ)[8,9].Hence,the short residence time of products in the channels of catalyst benefits the reduction of secondary reactions.Moreover,efficient gas–liquid mixing and high mass transfer are required for the hydrogenation rate,as catalytic hydrogenation of eAQ is a fast reaction and the reaction rate is controlled by mass transfer[10,11].
Monolith catalyst(structured catalyst)combining a large geometrical surface area with low pressure drop and a high degree of uniformity has been received growing attention in recent years[12].Many studies demonstrated that monolith catalyst is suitable for a catalytic process,which is characterized by high flowrates and limited by mass transfer,and a need for high selectivity[13–16],such as exhaust gas cleaning[17],catalytic combustion[18],alkylation of benzene with propylene[19],hydrodesulfurization[20,21],Fischer–Tropsch synthesis[22],catalytic cracking of hydrocarbon fuels[23,24]and selective hydrogenation[25–27].Irandoust et al.[28]investigated the influence of flow pattern(bubble flow and slug flow), flow distribution,pressure drop and mass transfer on the behaviors of monolithic three-phase reactors that were used in the hydrogenation step of AQs for the production of H2O2.Their results illustrated that slug flow gives the highest production rate with very small scale-up effects.Liu et al.[29]also studied the effects of flow state of reactants and hydrodynamics parameters on the conversion of the eAQ hydrogenation in a single square channel monolith reactor.Zhang et al.[30]and Li et al.[31]found that the average yield of H2O2on Pd/cordierite monolithic catalyst was higher than that on Pd/Al2O3pellet catalyst.Albers et al.[32]made a comparative study among slurry reactor,packed-bed reactor and monolith reactor for the production of H2O2by the AO process at their optimal operating conditions.It turned out that the monolith reactor obtained the high selectivity(low AQ losses)and the high reaction rate even after a long time on stream.
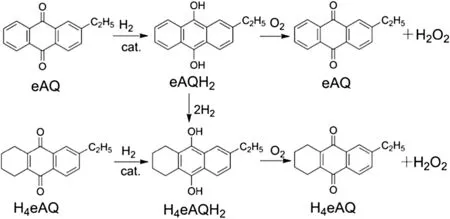
Fig.1.Simplified reaction pattern of the AOprocess.eAQ:2-ethyl-anthraquinone;eAQH2:2-ethyl-anthrahydroquinone;H4eAQ:5,6,7,8-tetrahydro-2-ethyl-anthraquinone;H4eAQH2:5,6,7,8-tetrahydro-2-ethyl-anthrahydroquinone.
The performance of monoliths is in fact limited by both monolithic catalyst and reactor technology.A typical monolithic catalyst consists of a support having sufficient mechanical strength,a porous layer providing the specific surface area enlargement that is needed for the catalysis and a catalytically active phase that is affected greatly by the structure of the porous layer.Apparently,high dispersion of catalytically active phase that can offer more active sites for chemical reactions is necessary.However,high dispersion will induce an increase of penetration depth of catalytically active compounds in the porous layer,which is unfavorable to the diffusion of reactants and products.As a result,the conversion and selectivity of the AQ hydrogenation would decrease.Drelinkiewicz et al.[33]found that the thickness of Pd shell is a variable essentially affecting the activity and life time of the alumina supported egg-shell Pd spherical catalysts,and there is an appropriate Pd shell thickness for maximizing the performance of the eAQ hydrogenation.A similar conclusion was reached by Zhang et al.[30].
The aim of this paper is to explore how the porous oxide layer on monolithic support affects the structure of Pd-based monolithic catalysts,which will determine their catalytic performance.In this work,Al2O3,SiO2and Al2O3‐SiO2were employed as porous layers due to their wide applications as supports of catalysts.Firstly,Pd-based monolithic catalysts with the various type and thicknesses of oxide layer on honeycomb cordierite monoliths were prepared.Secondly,physico chemical properties of the monolithic catalysts were characterized by inductively coupled plasma spectrometer,X-ray powder diffraction,scanning electron microscopy,nitrogen adsorption–desorption measurements and X-ray photoelectron spectroscopy.Finally,catalytic hydrogenation of 2-ethyl-9,10-anthraquinone(eAQ)was carried out in a flowing tubular reactor packed with Pd-based monolithic catalysts at 60°C and atmosphere pressure to evaluate their catalytic performance.
2.Experimental
2.1.Materials
Trioctyl phosphate(TOP,purity>96.0%)was purchased from Tokyo Chemical Industry(Shanghai)Co.Ltd.1,3,5-Trimethyl benzene(TMB,purity>98.0%),ethanol(purity>99.9%),p-xylene(purity>99.0%)and diethyl-o-phthalate(purity>99.0%)were purchased from Tianjin Guangfu Fine Chemical Research Institute.2-Ethyl-9,10-anthraquinone(eAQ,purity>99.0%)was purchased from DOW Chemical Company.PdCl2(purity>99.0%)was purchased from Tianjin Heowns Biochem LLC.Al2O3sol(20%in deionized water),SiO2sol(25%in deionized water)and SiO2‐Al2O3sol(24%in deionized water,Al2O3content of 1 wt%)were purchased from DaLian Snow Chemical Co.Ltd.Commercial honeycomb cordierite(2MgO·2Al2O3·5SiO2)monolith as support was purchased from Ping Xiang San Yuan Alvedate Ceramics Manufacture Co.Ltd.
The cordierite monolith was 50 mm in length,30 mm in outside diameter and 2 mm in average cell diameter,with straight and parallel channels of roughly triangle section and a cell density of 140 cpsi.Prior to use,the support was treated in p-xylene at 140°C for 20 min,washed with ethanol to remove residual p-xylene and calcined at 600°C in air for 2 h.Afterwards the support was treated sequentially with H2O2(30%)and NH3·H2O(30%).Finally,the pre-treated support was thoroughly cleaned with deionized water and dried in a stove at 120°C for 24 h.
2.2.Preparation of oxide layer
The oxide layers on cordierite monoliths were prepared with oxide sols by the dip-coating technique.Prior to the coating procedure,all the sols were diluted with deionized water(mass ratio of oxide sol to deionized water=1:1).After completion of the dip-coating procedure,the coated supports were dried at 120°C in air for 6 h and calcined at 600°C for5 h.The preparation procedure mentioned above was repeated until the mass loading of oxide layer reached a desired amount.The monolithic supports thus obtained are denoted as oxide/monolith(oxide=Al2O3,SiO2and SiO2‐Al2O3).
2.3.Deposition of Pd on oxide/monolith supports
The deposition of Pd on the oxide/monolith supports was performed using an impregnation method.Typically,the supports with oxide layers were immersed into a freshly prepared aqueous solution of Pd(NH3)2Cl2(by dissolving 34.0 mg PdCl2in 10 ml 30%NH3·H2O and then diluting with ca.220 ml deionized water to pH=11.5)at room temperature for 24 h.After deposition,the as-deposited monoliths were dried at 110 °C for 6 h and calcined at 350 °C for 2 h.Finally,the monolithic catalysts were reduced in H2flow(30 ml·min?1)at 80 °C for 1 h in a fixed-bed reactor.The as-prepared catalysts are denoted as MC series,including MC-Al(i.e.Pd/Al2O3/monolith),MC-Si(i.e.Pd/SiO2/monolith)and MC-Si–Al(i.e.Pd/SiO2‐Al2O3/monolith).
2.4.Characterization of oxide layers and monolithic catalysts
The adherence strength of oxide layer to monolithic support was tested by ultrasonic treatment.The ultrasonic treatment of oxide/monolith was carried out in an ultrasonic bath(65 kHz,20 kW) filled with ethanol at room temperature for 15 min.After treating,the monoliths with oxide layers were dried at80°C for 12 h and weighted.The Pd content of monolithic catalysts was determined using an inductively coupled plasma atomic emission spectrometer(ICP-9000(N+M),Thermo Jarrell-Ash Corp.).Firstly,the Pd contents in the freshly prepared aqueous solution of Pd(NH3)2Cl2and the remaining solution after deposition were determined,respectively.Then the amount of Pd loading on the catalysts was calculated from subtraction method.The Nitrogen adsorption–desorption isotherms were measured at 77 K on a volumetric adsorption analyzer(ASAP-2020,Micromeritics Instrument Corp.).X-ray diffraction patterns(XRD)were recorded on a Rigaku D-max 2500 diffractometer using CuKαradiation source(40 kV,200 mA)with a scan rate of 4(°)·min?1in the 2θ range from 30°to 80°.The surface morphology and thickness of oxide layers were measured by an environmental scanning electron microscopy(SEM,NanoSem 430 field emission gun scanning electron microscope,FEI Co.)equipped with an energy-dispersive X-ray analyzer(EDX).X-ray photoelectron spectroscopy measurement was carried out on a PHI 5000 X-ray photoelectron spectroscopy(XPS,PHI Co.).The samples used in nitrogen adsorption–desorption,XRD,SEM and XPS measurements were small pieces(about 3×1 mm)which were obtained by cutting away from the bulk monolithic catalysts.
2.5.Catalytic test
Catalytic hydrogenation of eAQ was carried out at 60°C and atmosphere pressure in a flowing reactor with an inside diameter of 30 mm and a length of 130 mm(Fig.2).Two Pd-based monolithic catalysts(MCs)were sealed in the middle part of the reactor.Both the upstream and downstream of catalysts were filled with glass beads of 3 mm in diameter to ensure the uniform flow of gas and liquid phases.The reaction temperature was controlled by water cycling in the jacket outside the reactor.Before the hydrogenation test the monolithic catalyst was activated“in-situ”inside the reactor by passing through the reactor hydrogen gas(30 ml(STP)·min?1)at 80 °C for 1 h.In a typical run,150 ml of the working solution consisting of a mixed solvent(TMB and TOP with the volume ratio of 65:35)and eAQ((0.32 ± 0.01)mol·L?1)was added to a reservoir.H2at flow rate of 30 ml(STP)·min?1controlled by a mass flow controller was continuously passed through a bubbler containing TMB,then to the top of the reactor.The role of the bubbler is to provide H2saturated with TMB for the reactor,preventing excessive evaporative loss of TMB from the vent.The working solution controlled at 50 ml·min?1by a high-pressure liquid chromatography pump was continuously fed to the top of the reactor.The mixture of gas and liquid phase in the outlet of the reactor was allowed to flow into a gas–liquid separator that was operated at 5°C.Gas was vented into the air and liquid was circulated back to the reservoir.The samples of the hydrogenated working solution were collected from the bottom of the separator under nitrogen protection.After that,the sample was oxidized with oxygen at atmospheric pressure and room temperature for about 20 min and the formed H2O2was then extracted with deionized water.The extraction was repeated four times and the aqueous solutions obtained thus were combined.Then,the content of H2O2in water phase was determined by titration with KMnO4solution.The organic phase(raffinate)was analyzed by a Bruker 456 gas chromatograph(Bruker Co.)with a flame ionization detector(FID)and a DB-17 column(60 m × 0.32 mm × 0.25 μm)at 260 °C with a heating rate of 2 °C·min?1and flow rate of H2gas at 1 ml·min?1.
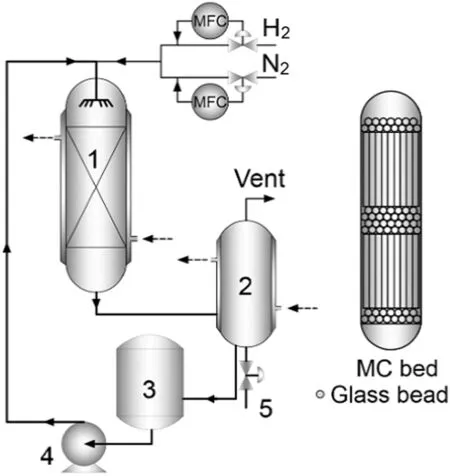
Fig.2.Schematic diagram of hydrogenation set-up.(1)Reactor;(2)Gas–liquid separator;(3)Feed reservoir;(4)Pump;(5)Sample connection.
To evaluate the catalyst stability,an additionalhydrogenation test of about 10 h was sequentially carried out over the catalyst without the treatment.In the second test,a freshly prepared working solution with the same composition was used.For each catalyst tested,three individual runs were performed to ensure the reproducibility of the experimental results.
The results of the experiments allowed calculation of the values of the following functions:the conversion of eAQ and hydrogenation efficiency(formed amount of H2O2per liter working solution,g·L?1).Since hydroquinone cannot be analyzed directly(easily oxidized by oxygen),the consumed amount of eAQ was calculated by Eqs.(1)and(2).The degradation products in Eq.(1)are the by-products that are not oxidized to form H2O2and thus represent a loss of starting eAQ.In this work,2-ethyl-anthrone(eAN)and 2-ethyl-1,2,3,4-tetrahydroanthraquinone were detected.
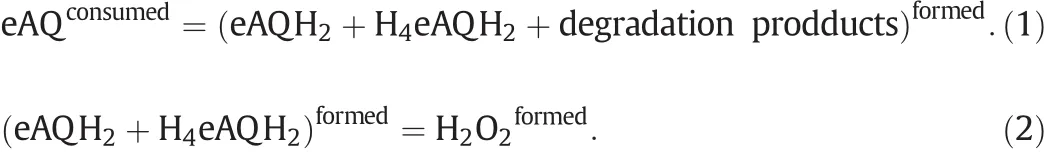
3.Results and Discussion
3.1.Characterization of oxide layers and monolithic catalysts
Table 1 summarizes the mass loading and adhesion of oxide layers,and content of Pd on cordierite monoliths.A wide variety of the mass loading of Al2O3layers were obtained by changing the coating times.For three different oxide layers,their mass loading is close.Moreover,the mass loss after adherence test by ultrasonic treatment for all oxidelayers is very low(less 1%),implying good adherence.From Table 1,it can be found that the content of Pd for monolithic catalysts(MCs)is almost the same,although they have different oxide layers or different thickness of Al2O3layers.
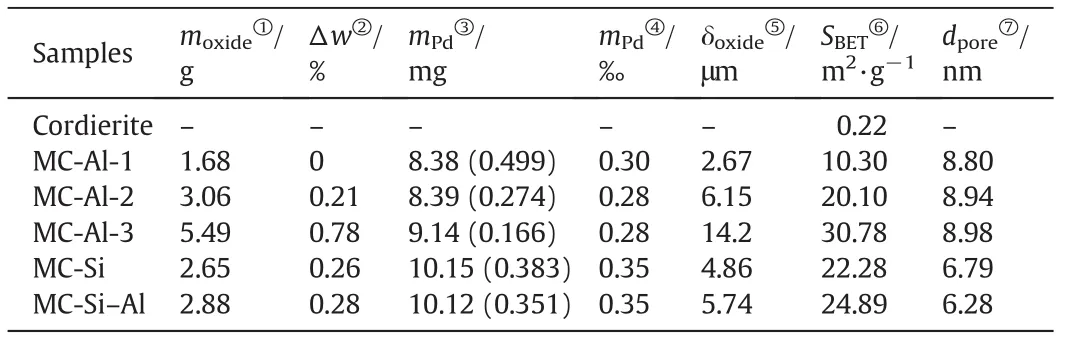
Table 1 Chemical compositions and textural properties of cordierite monolith and catalysts
Fig.3 shows the SEM micro graphs of the surface and cross-section of MCs.The surface morphology of MC-Al-1(Fig.3a)is rough and full of grooves,which is similar to that of cordierite monolith(Fig.3f).The surface morphology of MC-Al-3 appears uniform and smooth with few sinking pores.The surfaces of MC-Al-2,MC-Si and MC-Si–Al are similar and their rough degree is between those of MC-Al-1 and MC-Al-3.
The average thickness of the oxide layers of MC-Als increases,going from 2.67 to 14.2μm,butit does not linearly increase with mass loading of Al2O3layer due to the presence of a thin transitional layer between the cordierite monolith and oxide layer(as shown in Table 1 and Fig.3).Indeed,transitional layer between the cordierite monolith and oxide layer also existed in MC-Si and MC-Si–Al.Moreover,the MC-Al-2,MC-Si and MC-Si–Al have the similar thickness of about 5–6 μm.The results indicate that the thickness and mass loading of oxide layers could be controlled by varying the dip-coating times.
Fig.4 depicts the XRD patterns of MCs.As reference,the XRD patterns of Al2O3powder obtained from Al2O3sol through drying and calcination and bare cordierite monolith are also given in Fig.4 On the XRD patterns of cordierite monolith,there are several diffraction peaks at 2θ =33.9°,36.9°,43°,46.4°,54.3°and 69.7°,attributed to the(212),(220),(312),(320),(224)and(602)crystal faces of 2θ =37.7°,45.9°and 66.8°assigned to the(311),(400)and(440)crystal faces of γ-Al2O3phase.Forthree Pd/Al2O3/monolith catalysts(MC-Als),the characteristic diffraction peaks of both cordierite andγ-Al2O3were observed.With increasing the mass loading ofAl2O3,the peak intensity of cordierite phase gradually dropped,together with an enhancement in the peak intensity of γ-Al2O3phase.This indicates that γ-Al2O3layer was coated uniformly on the cordierite monolith and the thickness increased with increasing the mass loading of Al2O3,which is in good agreement with the observation by SEM.For MC-Si and MC-Si–Al,however,only characteristic diffraction peaks of cordierite were observed(not shown).This could be ascribed to that the SiO2and SiO2‐Al2O3layers on cordierite monoliths are amorphous.
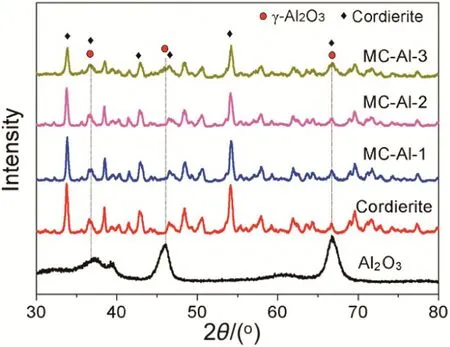
Fig.4.XRD patterns of monolithic catalysts,cordierite monolith and Al2O3 powder.
Further analysis of reduced and unreduced MCs was performed using XPS.The Pd(3d)spectra obtained from reduced and unreduced MC-Al-1 are shown in Fig.5.The binding energies(BE)of 335.1 and 340.1 eV correspond to the 3d5/2and 3d3/2states of Pd0species.The similar energies of 335.0 and 340.1 eV for reduced MC-Al-1 indicate that Pd0species existed on it.Unreduced MC-Al-1 shows the binding energies of 341.6 and 336.3 eV,which is characteristic of PdO.Additionally,two weak peaks at the binding energies(Eb)of 336.7 and 341.2 eV,respectively,can be observed in Fig.5(a),indicating the presence of PdO in reduced MC-Al.It mightbe ascribed to oxidation of the reduced sample in air during measurement of XPS.The slight difference in the value of Eb(Pd3d5/2)is due to the measurement error.I vanova et al.reported Eb(Pd3d5/2)=336.3–336.8 eV for PdO[34].However,there were no any signals of metallic or oxidized Pd species being detected in other monolithic catalysts whether they were reduced or not.It could be explained by the different thicknesses of oxide layers which results in the different loadings of Pd in oxide layer,although the total content of Pd in catalysts is almost the same.MC-Al-1 has the thinnest oxide layer among all MCs.Accordingly,the loading of Pd in its oxide layer is highest(0.499%,in Table 1).
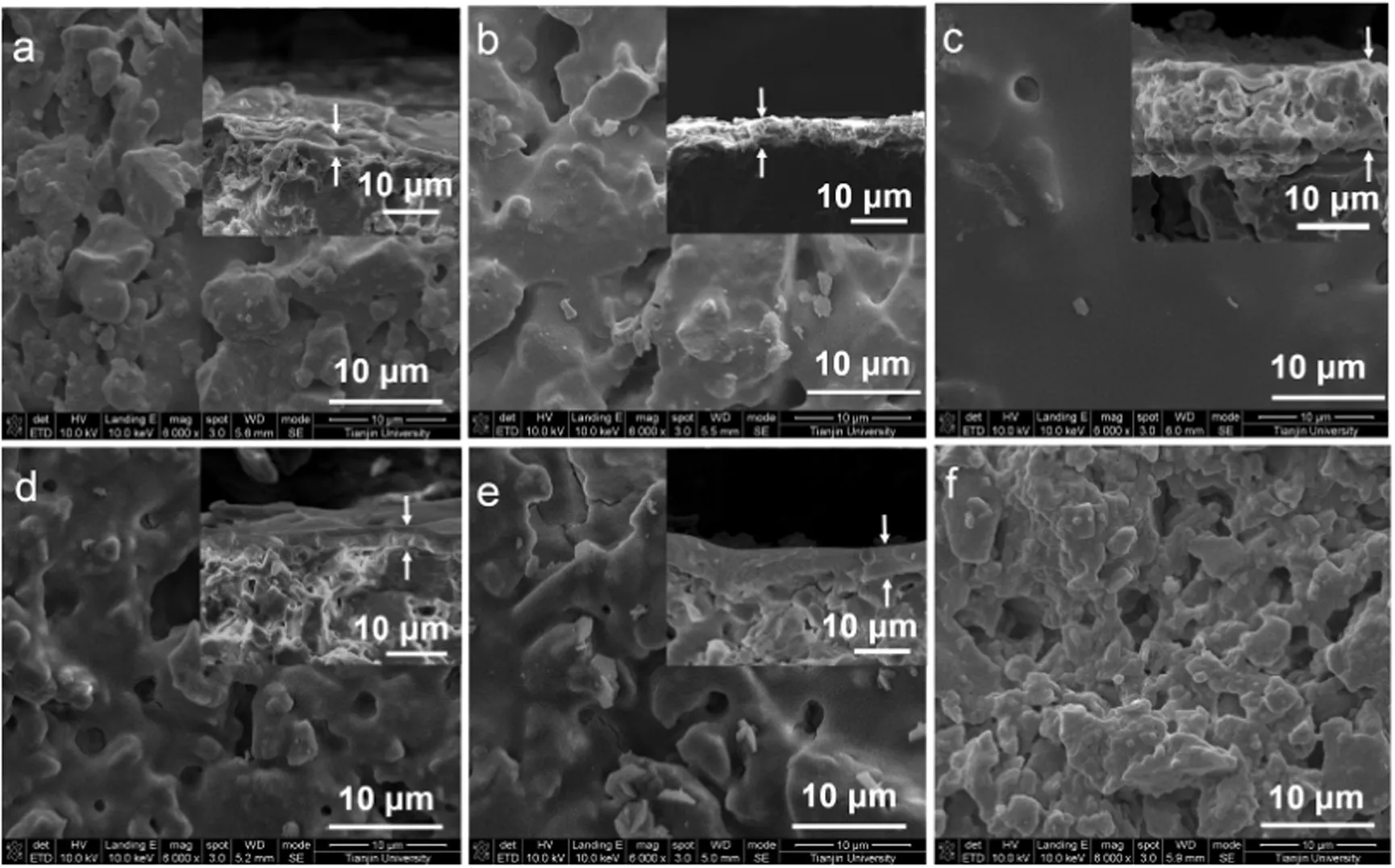
Fig.3.SEM images of monolithic catalysts a–e:top view of MC-Al-1,MC-Al-2,MC-Al-3,MC-Si and MC-Si–Al(inserts are their corresponding section),f:top view of bare cordierite monolith.
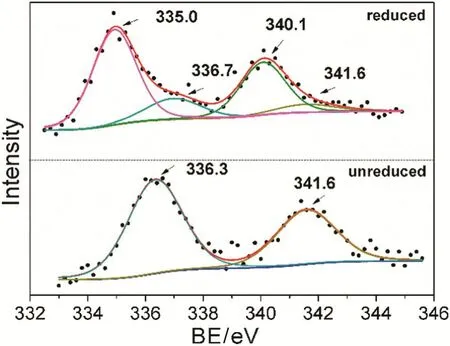
Fig.5.XPS spectra of MC-Al-1.
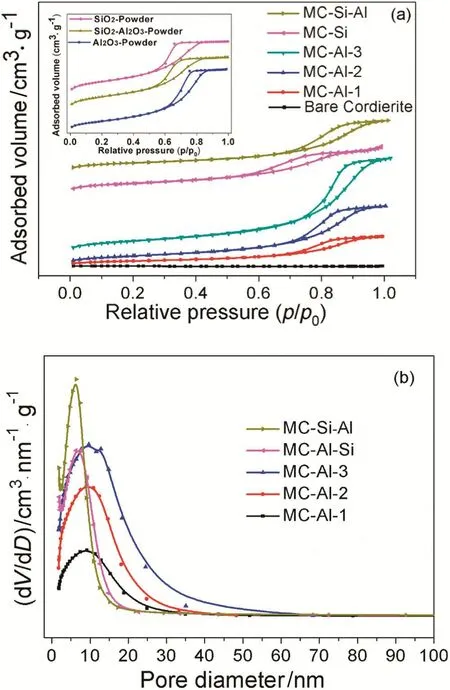
Fig.6.(a)N2 adsorption–desorption isotherms(inserts are corresponding oxide powder)and(b)pore size distributions of cordierite monolith and MCs.
Fig.6 displays the N2adsorption–desorption isotherms and the pore size distribution curves of cordierite monolith and Pd/oxide/monolithic catalysts(MCs),as well as Al2O3,SiO2and Al2O3‐SiO2powder that were obtained from corresponding oxide sol by drying and calcination.The corresponding textural properties are summarized in Table 1.As shown in Fig.6(a),all samples show typical type IV isotherms with H2-shaped capillary condensation loop which is indicative of channel-like or ink-bottle mesopores[35].However,the relative pressure(p/p0)range ofhysteresis loop differs from each other.Compared to Al2O3powder(hysteresis loop at p/p0=0.6–0.85),MC-Als present the high pressure hysteresis loop(p/p0=0.7–0.95),indicating the presence of nonuniform mesoporous accumulation pores.A similar phenomenon could be observed on the adsorption–desorption isotherms of MC-Si–Al and SiO2‐Al2O3powder.For MC-Si,the hysteresis loop is in the range of p/p0=0.55–0.8,which is in good agreement with that for SiO2powder.This indicates that there is no accumulation mesopores existed in MCSi.In Fig.6(b),MC-Als exhibit mesopores with the pore size centered around 9 nm and the amount of their mesopores increases with the mass loading of Al2O3layers.MC-Si and MC-Si–Al present mesopores with the size centered around 6.5 nm,which is ca.30%smaller than MC-Als.
From Table 1,it can be seen that bare cordierite monolith has an extremely small specific surface area(0.22 m2·g?1).After coating the oxide layer,the specific surface area increases rapidly(between about 10 and 30 m2·g?1).Moreover,for MC-Al series,the specific surface area becomes significantly bigger with increasing the mass loading of Al2O3layers due to the increase in the fraction of oxide layer in catalysts.For three monolithic catalysts with the similar mass loading and thickness of oxide layer,their specific surface areas are close.
3.2.Catalytic performance
3.2.1.Effect of oxide on conversion of eAQ
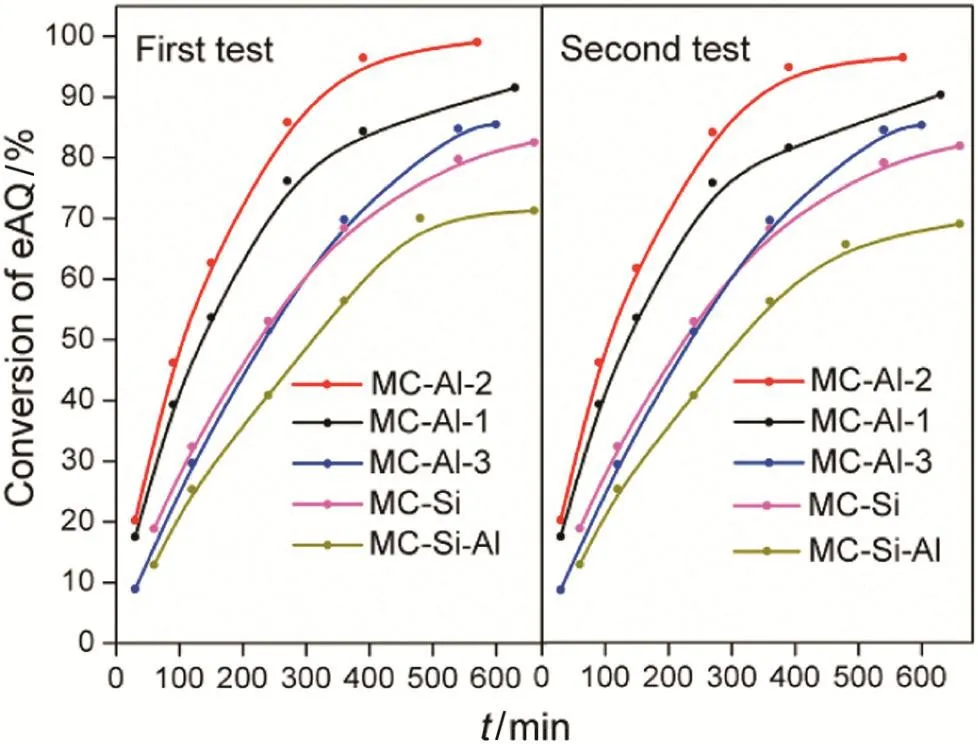
Fig.7.Conversion of eAQ over monolithic catalysts versus reaction time.
Catalytic hydrogenation of eAQ was carried in a flowing tube reactor in which the as-prepared monolithic catalysts(MCs)were packed.Fig.7 shows the conversion of eAQ catalyzed by various monolithic catalysts as a function of time on stream(TOS).For allcatalysts,two tests gave almost the same conversion of eAQ,indicating good catalyst stability.In Fig.7,the conversion of eAQ increased quickly with the going on of reaction,especially at the initial stage of hydrogenation.Generally,the monolithic catalysts on Al2O3layers(MC-Als)exhibited the superioractivity.For instance,the conversion of eAQ over MC-Al-2 reached 99.1%at 570 min,which is about 1.3 times higher than those of MC-Si and MC-Si–Al with the similar mass loading of oxide layer.It could be explained by difference in the pore size of different oxide layers,the relatively large pore size of MC-Als could improve effective diffusivity of the reactant molecules and thus bring benefits for catalytic activity.Shang et al.prepared a new egg-shell palladium catalyst with Racshig-ring alumina as support and found that it has the higher hydrogenation activity of eAQ compared to sphere-and four-leaf clover-shaped commercial catalysts.Authors came to the conclusion thatit was attributed to larger pore diameter and higher voidage which can efficiently reduce the internal and external diffusion resistance[36].Drelinkiewicz et al.[7]studied the effects of the type of support(SiO2(1),SiO2(2)and Al2O3)on the performance of supported egg-shell catalysts in the hydrogenation of eAQ.They found that two silica supported catalysts exhibited much better activity and activity maintenance than alumina supported catalysts,and the initial activity of SiO2(2)supported catalysts was much higherthan those ofSiO2(1)based ones.In other words,the initial activity of silica and alumina supported catalysts has the same trend as their average pore diameter(13.3 nm for SiO2(2),10.6 nm for SiO2(1)and 5.32 nm for Al2O3).Feng et al.[37]prepared Pd/Al2O3and Pd/SiO2‐Al2O3catalysts and found that the reaction activity and stability in the hydrogenation of eAQ increased with increasing silica content from 2 wt%to 8 wt%.Authors attributed those to the increase in surface acidity and specific surface area with the addition of silica,which led to the smaller Pd crystallite size,higher Pd dispersion and lower diffusion resistance.
The thickness of γ-Al2O3layer is also a crucial variable affecting the catalytic performance of monolithic catalysts.Among MC-Als,one with middle thickness of Al2O3layer exhibits the highest conversion of eAQ.This indicates that a suitable thickness of γ-Al2O3layer is favorable to the catalyst activity.It is generally accepted that high dispersion of active compounds and rapid diffusion of reactants inside the pore channels of catalysts have a positive effect on catalytic activity.The thinner γ-Al2O3layer provides the low dispersion of Pd on support,resulting in less catalytically active sites.But,the thicker γ-Al2O3layer causes the Pd penetrating depth on support to be deeper which limits diffusion of reactants,resulting in the activity of catalyst being not fully exploited,i.e.the low catalyst utilization.
To getmore insights into the role of oxide layer on the distribution of Pd,the distribution of Pd along Al2O3layer was measured by SEM–EDX and the results are shown in Fig.8.It reveals that Pd distributed mainly in the oxide layer.In the transition all ayer bet ween the cordierite monolith and oxide layer,a small amount of Pd was detected,but there was no Pd being detected in cordierite monolith.With increasing the thickness of oxide layers,the distribution of Pd in oxide layer becomes more uniform.MC-Al-1 gave the narrowest distribution of Pd(about 2 μm below the top surface)because of the thinnest thickness of layer,which led to a remarkably high content of Pd on its near top surface.It is the reason for that Pd on MC-Al-1 was detected by XPS because this technique is highly surface specific.Among MC-Als,MC-Al-2 has the moderate Pd penetrating depth,which provides a balance between the distribution of Pd and accessibility of active sites by reactant molecules.Similar distribution of Pd is found among MC-Al-2,MC-Si and MC-Si–Al,supporting further the speculation of the different pore sizes between MC-Als and MC-Sis(MC-Si and MC-Si–Al)as a main reason for their differences in the activity.
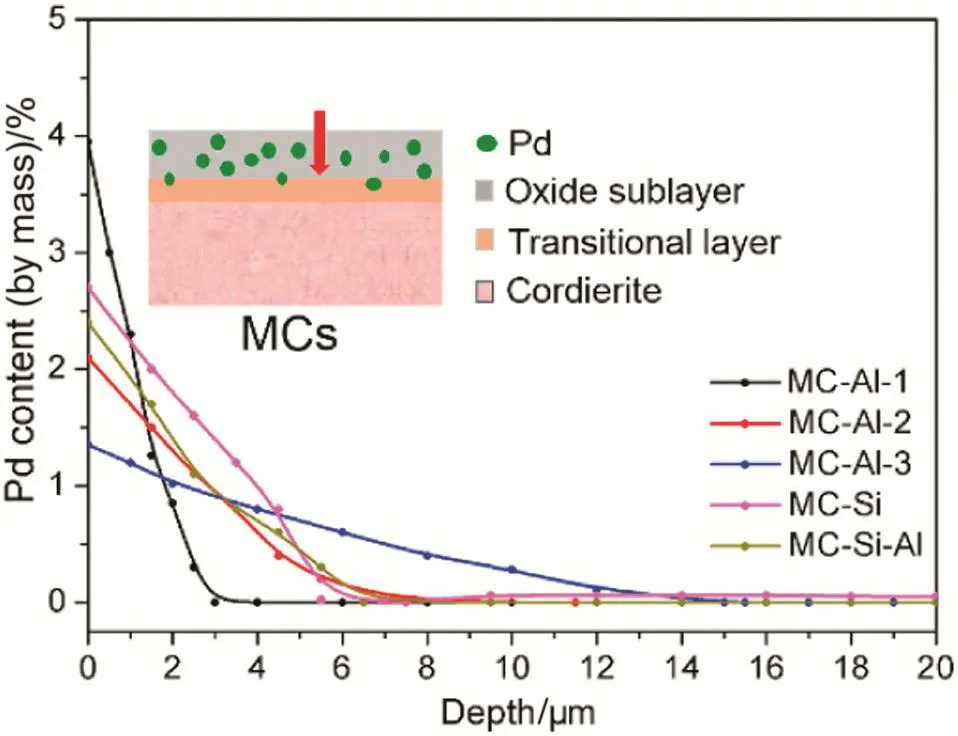
Fig.8.SEM–EDX spectra of monolithic catalysts.
3.2.2.Effect of oxide sublayer on hydrogenation efficiency
Fig.9 shows hydrogenation efficiency over monolithic catalysts as a function of TOS.Overall,the trend of hydrogenation efficiency obtained over various catalysts is in good agreement with their conversion of eAQ.MC-Al-2 presents the highest hydrogenation efficiency up to 10.0 g·L?1at 570 min among all the catalysts.The thickness of Al2O3layer on MC-Al-2 is 6 μm,which is much lower than that reported by Zhang et al.[30]whose optimal Al2O3washcoat is about 38 μm.The difference was possibly caused by the different compositions of the working solution.The mixed solvent of TMB and TOP with the volume ratio of 3:1 and the eAQ concentration of 60 g·L?1were used by Zhang et al.,whereas the mixed solvent of TMB and TOP with the volume ratio of 65:35 and the eAQ concentration of 75 g·L?1were used in our work.Since viscosity of TOP[38](14.087 mPa·s at 25 °C)is much higher than that of TMB[39](0.658 mPa·s at 25 °C)and a relatively high concentration of solute,viscosity of the working solution used in this work is much higher.Under such situations,hydrogenation reaction would encounter more severe diffusion limitation.
It can be seen in Fig.9 that there was no discernible difference in hydrogenation efficiency between two tests being observed over all catalysts.Li et al.[31]compared the stability of Pd/Al2O3/cordierite(PAC)and egg-shell Pd/SiO2/cordierite(PSC)monolith catalyst for the eAQ hydrogenation.In their case the hydrogenation efficiency(5 g·L?1)over PSC starts to decline only after about 80 h on stream,while the hydrogenation efficiency over PAC falls from about5.6 to about1.2 g·L?1in merely 100 h on stream,that is,PAC has a slightly high initial activity and PSC showed much bet teractivity maintenance than PAC.Authors pointed out that the weak acidity of the PSC catalyst contributed to improving the catalyst stability.We noted that PAC has the much smaller pore diameter than PSC(2.2 nm for PAC and 7.8 nm for PSC),which cannot be excluded as an important reason for the extremely low activity stability.In our work,the pore diameter of MC-Als is larger than those of MC-Si and MC-Si–Al,which might be the reason for their high stability.Additionally,the relatively short reaction test time(20 h)might be insufficient to obtained deactivation behavior.The extended catalyst tests up to at least more than 20 h are needed,before we can speculate further.
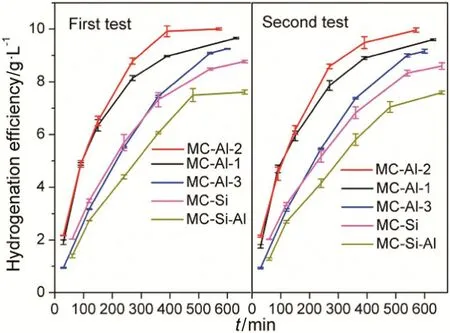
Fig.9.Hydrogenation efficiency over monolithic catalysts versus reaction time.
4.Conclusions
Pd-based monolithic catalysts were prepared on honeycomb cordierite monoliths and tested for the eAQ hydrogenation.The type and thickness of oxide layer were shown to be the crucial variables affecting the catalytic performance.The monolithic catalysts with γ-Al2O3as layers showed the high catalytic performance compared to ones with SiO2and SiO2‐Al2O3layers.Moreover,an increase in the thickness of Al2O3layer could increase the Pd penetrating depth in catalyst,which benefits its catalytic performance.But,the performance of monolithic catalysts did not vary monotonously with the thickness of Al2O3.The catalyst with the suitable thickness ofγ-Al2O3sublayer(about6μm)exhibits the high conversion of eAQ and hydrogenation efficiency.Values of 99.1%and 10.0 g·L?1respectively can be achieved.
[1]V.Russo,R.Tesser,E.Santacesaria,M.Di Serio,Chemical and technical aspects of propene oxide production via hydrogen peroxide(HPPO process),Ind.Eng.Chem.Res.52(3)(2013)1168–1178.
[2]A.Okninski,B.Bartkowiak,K.Sobczak,D.Kublik,P.Surmacz,G.Rarata,B.Marciniak,P.Wolanski,Development of a small green bipropellant rocket engine using hydrogen peroxide as oxidizer,50th AIAA/ASME/SAE/ASEE joint propulsion conference,American,2014.
[3]H.M.Chen,D.P.Huang,X.Y.Su,J.L.Huang,X.L.Jing,M.M.Du,D.H.Sun,L.S.Jia,Q.B.Li,Fabrication of Pd/γ-Al2O3catalysts for hydrogenation of 2-ethyl-9,10-anthraquinone assisted by plant-mediated strategy,Chem.Eng.J.262(2015)356–363.
[4]Y.X.Cheng,L.Wang,S.X.Lü,Y.Q.Wang,Z.T.Mi,Gas–liquid–liquid three-phase reactive extraction for the hydrogen peroxide,Ind.Eng.Chem.Res.47(2008)7414–7418.
[5]G.Z.Liu,Y.Duan,Y.Q.Wang,L.Wang,Z.T.Mi,Periodically operated trickle-bed reactor for EAQs hydrogenation:Experiments and modeling,Chem.Eng.Sci.60(22)(2005)6270–6278.
[6]J.Petr,L.Kurc,Z.Bělohlav,L.?erveny,Catalytic hydrogenation of 2-ethyl-9,10-anthrahydroquinone,Chem.Eng.Process.43(7)(2004)887–894.
[7]A.Drelinkiewicz,A.Pukkinen,R.Kangas,R.Laitinen,Hydrogenation of 2-ethylanthraquinone over Pd‐SiO2and Pd‐Al2O3in the fixed-bed reactor.The effect of the type of support,Catal.Lett.94(2004)157–170.
[8]A.Drelinkiewicz,Kinetic aspects in the selectivity of deep hydrogenation of 2-ethylanthraquinone over Pd--SiO2,J.Mol.Catal.A Chem.101(2004)61–74.
[9]R.Kosydar,A.Drelinkiewicz,J.P.Ganhy,Degradation reactions in anthraquinone process of hydrogen peroxide synthesis,Catal.Lett.139(3–4)(2010)105–113.
[10]E.Santacesaria,M.Di Serio,A.Russo,U.Leone,R.Velotti,Kinetic and catalytic aspects in the hydrogen peroxide production via anthraquinone,Chem.Eng.Sci.54(1999)2799–2806.
[11]J.Tan,J.S.Zhang,Y.C.Lu,J.H.Xu,G.S.Luo,Process intensification of catalytic hydrogenation of ethylanthraquinone with gas–liquid microdispersion,AIChE J 58(5)(2012)1326–1335.
[12]C.N.Dai,Z.G.Lei,Y.L.Wang,R.D.Zhang,B.H.Chen,Transfer and reaction performances of selective catalytic reduction of N2O with CO over monolith catalysts,Chin.J.Chem.Eng.21(8)(2013)835–843.
[13]D.I.Enache,P.E.Landon,M.V.Lok,S.D.Pollington,E.H.Stitt,Direct comparison of a trickle bed and a monolith for hydrogenation of pyrolysis gasoline,Ind.Eng.Chem.Res.44(2005)9431–9439.
[14]R.M.Machado,R.R.Broekhuis,A.F.Nordquist,B.P.Roy,S.R.Carney,Applying monolith reactors for hydrogenations in the production of specialty chemicals-process and economic considerations,Catal.Today 105(3–4)(2005)305–317.
[15]S.Roy,T.Bauer,M.Al-Dahhan,P.Lehner,T.Turek,Monoliths as multiphase reactors:A review,AIChE J 50(2004)2918–2938.
[16]S.Roy,A.K.Heibel,W.Liu,T.Boger,Design of monolithic catalysts for multiphase reactions,Chem.Eng.Sci.59(5)(2004)957–966.
[17]P.Avila,M.Montes,E.E.Miró,Monolithic reactors for environmental applications,Chem.Eng.J.109(1–3)(2005)11–36.
[18]J.C.Zhou,D.F.Wu,W.Jiang,Y.D.Li,Catalytic combustion of toluene over a copper–manganese–silver mixed-oxide catalyst supported on a washcoated ceramic monolith,Chem.Eng.Technol.32(10)(2009)1520–1526.
[19]C.N.Dai,Z.G.Lei,J.Zhang,Y.X.Li,B.H.Chen,Monolith catalysts for the alkylation of benzene with propylene,Chem.Eng.Sci.100(2013)342–351.
[20]Q.Y.Li,P.Y.Wu,L.Lan,H.Liu,F.Ji,The effect of Ca on structure and performance of hydrodesulfurization of Ca‐Ni2P/SBA-15/cord monolithic catalysts,Catal.Today 216(2013)38–43.
[21]N.Wei,S.F.Ji,P.Y.Wu,Y.A.Guo,H.Liu,J.Q.Zhu,C.Y.Li,Preparation of nickel phosphide/SBA-15/cordierite monolithic catalysts and catalytic activity for hydrodesulfurization of dibenzothiophene,Catal.Today 147(2009)S66–S70.
[22]W.Liu,Y.Wang,W.Wayne,L.Shari,A compact and high throughput reactor of monolithic-structured catalyst bed for conversion of syngas to liquid fuels,AIChE J 58(9)(2012)2820–2829.
[23]L.Wang,D.D.Zhao,S.J.Pu,G.Z.Liu,Remarkable catalytic activities of hydrothermally synthesized bilayered HZSM-5 coatings with additional nonzeolite pores,Ind.Eng.Chem.Res.54(19)(2015)5246–5253.
[24]M.L.Ji,G.Z.Liu,L.Wang,X.W.Zhang,Controllable fabrication and catalytic activity of highlyb-oriented HZSM-5 coatings,AIChE J 60(6)(2014)1964–1968.
[25]A.Devard,M.A.Ulla,F.A.Marchesini,Synthesis of Pd/Al2O3coating onto a cordierite monolith and its application to nitrite reduction in water,Catal.Commun.34(2013)26–29.
[26]Z.W.Liu,Q.F.Zhu,N.Hou,Y.Fu,L.X.Wen,J.F.Chen,Development of novel monolithic catalyst with porous hollow silica nanoparticles for selective hydrogenation reactions,Catal.Today 216(2013)205–210.
[27]J.Zhu,F.Wu,M.S.Li,J.J.Zhu,J.G.van Ommen,L.Lefferts,Influence of internal diffusion on selective hydrogenation of 4-carboxybenzaldehyde over palladium catalysts supported on carbon nanofiber coated monolith,Appl.Catal.A Gen.498(2015)222–229.
[28]S.Irandoust,B.Andersson,E.Bengtsson,M.Siverstrom,Scaling up of a monolithic catalyst reactor with two-phase flow,Ind.Eng.Chem.Res.28(1989)1489–1493.
[29]D.S.Liu,J.G.Zhang,D.F.Li,Q.D.Kong,T.Zhang,S.D.Wang,Hydrogenation of 2-ethylanthraquinone under Taylor flow in single square channel monolith reactors,AIChE J 55(3)(2009)726–736.
[30]J.G.Zhang,D.F.Li,Y.J.Zhao,Q.D.Kong,S.D.Wang,A Pd/Al2O3/cordierite monolithic catalyst for hydrogenation of 2-ethylanthraquinone,Catal.Commun.9(15)(2008)2565–2569.
[31]X.T.Li,H.J.Su,G.Y.Ren,S.D.Wang,A highly stable Pd/SiO2/cordierite monolith catalyst for 2-ethyl-anthraquinone hydrogenation,RSC Adv.5(122)(2015)100968–100977.
[32]R.E.Albers,M.Nystr?m,M.Siverstr?m,A.Sellin,A.-C.Dellve,U.Andersson,W.Herrmann,T.Berglin,Development of a monolith-based process for H2O2production-from idea to large-scale implementation,Catal.Today 69(2001)247–252.
[33]A.Drelinkiewicz,R.Kangas,R.Laitinen,A.Pukkinen,J.Pursiainen,Hydrogenation of 2-ethylanthraquinone on alumina-supported palladium catalysts,Appl.Catal.A Gen.263(1)(2004)71–82.
[34]A.S.Ivanova,E.M.Slavinskaya,R.V.Gulyaev,V.I.Zaikovskii,O.A.Stonkus,I.G.Danilova,L.M.Plyasova,I.A.Polukhina,A.I.Boronin,Metal–support interactions in Pt/Al2O3and Pd/Al2O3catalysts for CO oxidation,Appl.Catal.B Environ.97(1–2)(2010)57–71.
[35]M.Thommes,K.A.Cychosz,Physical adsorption characterization of nanoporous materials:Progress and challenges,Adsorption 20(2–3)(2014)233–250.
[36]H.Shang,H.J.Zhou,Z.H.Zhu,W.H.Zhang,Study on the new hydrogenation catalyst and processes for hydrogen peroxide through anthraquinone route,J.Ind.Eng.Chem.18(5)(2012)1851–1857.
[37]J.T.Feng,H.Y.Wang,G.E.David,X.Duan,D.Q.Li,Catalytic hydrogenation of ethylanthraquinone over highly dispersed eggshell Pd/SiO2‐Al2O3spherical catalysts,Appl.Catal.A Gen.382(2)(2010)240–245.
[38]F.Sheng,C.X.Zhao,C.H.He,J.Q.Liu,J.H.Sun,Densities and viscosities of binary mixtures of tris(2-ethylhexyl)phosphate+cyclohexane or n-hexane at T=(293.15,298.15,and 303.15)K and p=0.1 MPa,J.Chem.Eng.Data 53(2008)2718–2720.
[39]M.I.Aralaguppi,C.V.Jadar,T.M.Aminabhavi,Density,refractive index,viscosity,and speed of sound in binary mixtures of cyclohexanone with benzene,methylbenzene,1,4-dimethylbenzene,1,3,5-trimethylbenzene,and methoxybenzene in the temperature interval(298.15 to 308.15)K,J.Chem.Eng.Data 44(1999)446–450.
 Chinese Journal of Chemical Engineering2016年11期
Chinese Journal of Chemical Engineering2016年11期
- Chinese Journal of Chemical Engineering的其它文章
- COSMO-RS:An ionic liquid prescreening tool for gas hydrate mitigation☆
- Synthesis,characteristics of hierarchical EU-1 zeolite for xylene isomerization probe reaction☆
- Design and control of methyl acetate-methanol separation via heat-integrated pressure-swing distillation
- Solving chemical dynamic optimization problems with ranking-based differential evolution algorithms☆
- An analysis of an ethanol-based,whole-crop refinery system in China☆
- Simultaneous hybrid modeling of a nosiheptide fermentation process using particle swarm optimization☆
