Budd-Chiari syndrome in children: Challenges and outcome
Arghya Samanta,Moinak Sen Sarma,Rajanikant Yadav
Abstract Budd-Chiari syndrome (BCS) is an uncommon disease of the liver,characterised by obstruction of the hepatic venous outflow tract.The etiological spectrum of BCS as well as venous obstruction pattern show wide geographical and demographic variations across the globe.Compared to adults with BCS,children have primary BCS as the predominant etiology,earlier clinical presentation,and hence better treatment outcome.Underlying prothrombotic conditions play a key role in the etiopathogenesis of BCS,though work-up for the same is often unyielding in children.Use of next-generation sequencing in addition to conventional tests for thrombophilia leads to better diagnostic yield.In recent years,advances in radiological endovascular intervention techniques have revolutionized the treatment and outcome of BCS.Various non-invasive markers of fibrosis like liver and splenic stiffness measurement are being increasingly used to assess treatment response.Elastography techniques provide a novel non-invasive tool for measuring liver and splenic stiffness.This article reviews the diagnostic and therapeutic advances and challenges in children with BCS.
Key Words: Budd-Chiari syndrome;Radiological endovascular intervention;Transjugular intrahepatic porto-systemic shunt;Direct intrahepatic porto-systemic shunt;Liver stiffness;Splenic stiffness;Shear-wave elastography
INTRODUCTION
Budd-Chiari syndrome (BCS) is a rare vascular disease of the liver with a promising outcome if treated optimally on time.It occurs due to obstruction in the hepatic venous outflow anywhere from the hepatic vein (HV) to the entry of the inferior vena cava (IVC) to the heart[1].Involvement of at least two HV leads to an increase in hepatic sinusoidal pressure and congestion resulting in symptoms of BCS[2].Global epidemiological data on BCS is scarce[3].The incidence of BCS reported in the published literature ranges from 0.2 to 4.1 cases per million population per year,with an estimated prevalence of 2.4-7.7 per million population in Asian countries[4,5] and of 1.4-4.0 per million population in Western countries[6-9].Population-based study on the epidemiology of BCS in children is lacking.Large case series on pediatric BCS are available[10-15].Chronic BCS constitutes 3%-7% of cases of portal hypertension (PHTN) in the pediatric population[10,11].So far,interventional and long-term outcome studies in children are limited[12-15].Over the years there has been remarkable progress in understanding the evolution of this disease.This review aims to discuss the various aspects of BCS,including recent updates on diagnostic and treatment modalities for BCS in children as well as the challenges in them.The review will particularly focus on chronic BCS which accounts for the majority of cases in children.
VARIATIONS IN THE ETIOLOGICAL SPECTRUM OF BCS
Primary BCS is an obstruction of the hepatic venous outflow tract that occurs due to an endoluminal venous lesion(thrombosis or web) resulting from an unidentifiable cause or an inherent prothrombotic condition.Secondary BCS results from obstruction from an invasive lesion (malignant tumor or a parasitic mass) or extrinsic compression by spaceoccupying lesions (abscesses,cysts,and benign or malignant solid tumors)[1].The above inflammatory or neoplastic conditions can also result in a secondary prothrombotic state which further adds to thrombosis in the HV.Secondary causes of BCS are common in adults as compared to children[16].Global variations in the type of BCS may reflect different predisposing factors in different countries.Studies from Western countries have shown HV to be the most common site of obstruction in BCS[17,18].There seems to be a shift in the pattern of obstruction in studies from Asian countries.Earlier,isolated IVC obstruction was the most type of BCS in Asian patients.Recent studies from China[19,20]as well as India[16,21,22] have noted that a combined IVC and HV obstruction (40%-75%) is the most common type of BCS.A similar pattern has also been documented in Indian children with BCS[12-15].Over the years,the changes in the spectrum have been better documented due to the advancements in radiology such as high-resolution Doppler ultrasonography (DUS) and non-invasive venography by computed tomography (CT),and magnetic resonance imaging.
BCS AND THROMBOPHILIA
Primary BCS is often regarded as a result of a unique constellation of prothrombotic conditions.The inherited prothrombotic conditions associated with BCS are protein C deficiency,protein S deficiency,antithrombin III deficiency,factor V Leiden mutation,prothrombin gene mutation,and hyper-homocysteinemia with methyltetrahydrofolate reductase mutation[23].Acquired prothrombotic conditions like antiphospholipid syndrome (APLS),paroxysmal nocturnal hemoglobinuria (PNH),sickle cell disease,and myeloproliferative disorders (MPD) also predispose to BCS[24,25].Systemic disorders like inflammatory bowel disease,Behcet’s disease,and other inflammatory intraabdominal lesions can cause BCS in adults but rarely in children[26].Recent studies reported an identifiable etiology in 80-84% of cases in adults[27-30].Underlying myeloproliferative disorders (45%-51%) and exposure to oral contraceptives (50%) are the commonest etiologies[27,28].In over 25% of cases,more than one thrombophilic state may be present[29,30].From the available pediatric literature,we understand that the prothrombotic workup in children is often unyielding,inconclusive,or ambiguous.Table 1 summarizes the findings of prothrombotic workup of prior pediatric studies on BCS[11-14,31-35].As shown,most of the pediatric publications are from India including authors’ own experience.One of the majordrawbacks is the lack of in-depth testing for prothrombotic conditions.Shuklaet al[32] found thrombophilia in 42% of children and 37% of adolescents.The commonest etiologies were Factor V Leiden in children and JAK 2V617F mutation in adolescents.Although thrombophilia may be present in 68%-75% of children,the cause-and-effect relationship is not established[12,32].Relative and multiple deficiencies of proteins C and S,antithrombin III deficiency,and hyperhomocysteinemia (9%-15%) may be a result of an advanced liver disease resulting in poor synthetic function of the liver rather than a true thrombophilia state[29].In a systematic review of adults with BCS,MPD was found to be more associated with HV block (16%-62%) than IVC block (4%-5%)[36].
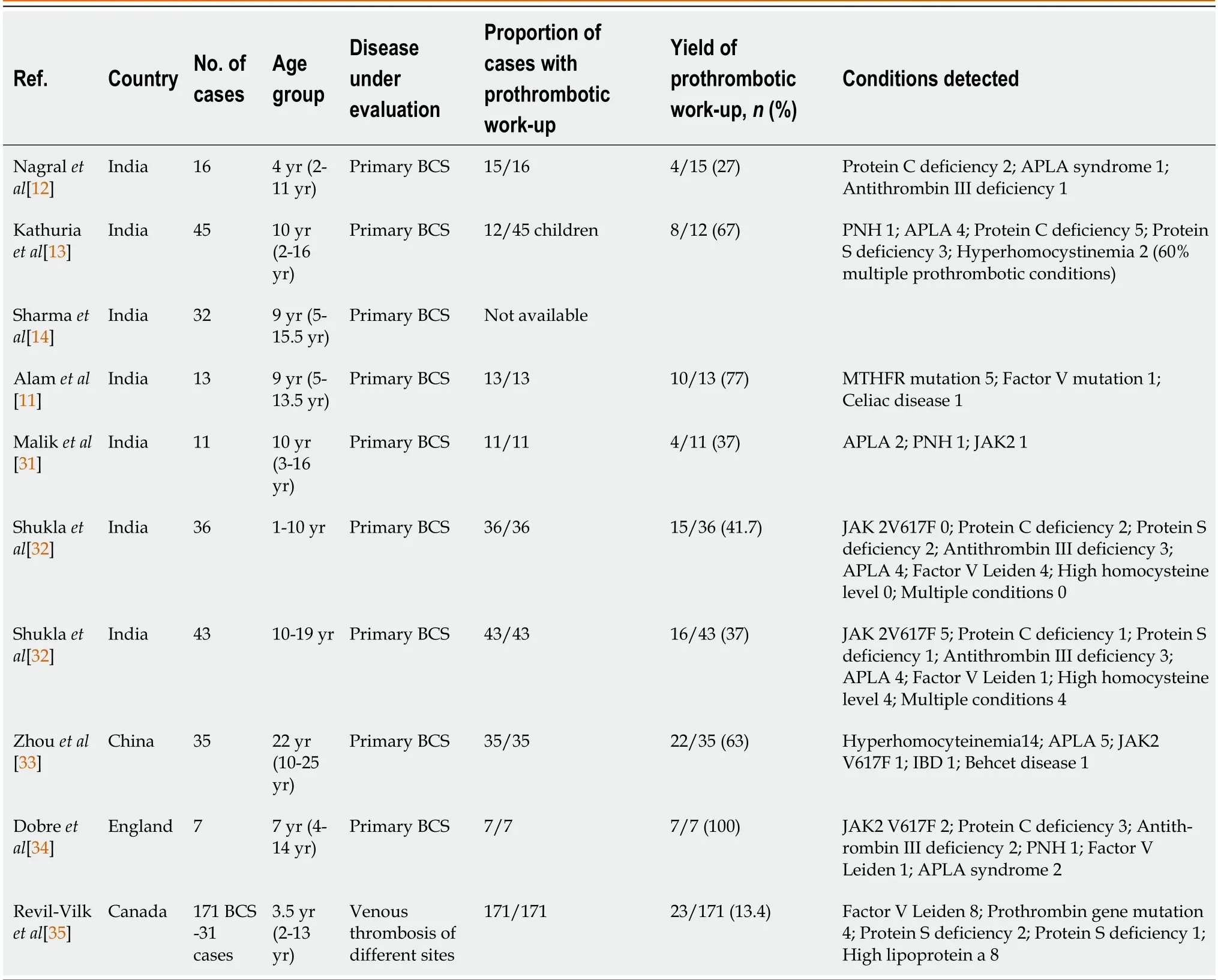
Table 1 Summary of various pediatric studies (national) of thrombophilia profile in children with Budd-Chiari syndrome
Oral anticoagulants may alter proteins C and S,activated protein C levels,and lupus anticoagulant levels.Hence these tests are best performed before starting thromboprophylaxis.Further,the presence of hypersplenism and hemodilution in cirrhosis with portal hypertension masks the peripheral blood findings of a concomitant MPD[29].Genetic testing is the gold standard for documentation of thrombophilia.In a study of 80 adults with non-cirrhotic splanchnic vein thrombosis,next-generation sequencing was able to identify JAK2 mutations,previously undetected by conventional techniques in one-third of cases[37].
It is also suggested that the prevalence of various thrombophilic disorders is different between younger children and adolescents.In a study from India,the JAK2V617F was found to be common in adolescents but not in children[32].Recently it has been shown that adolescent venous thromboembolism is multifactorial in the majority with more than two risk factors at diagnosis[38].Warfarin is the most commonly used oral anticoagulant in BCS patients,especially after radiological intervention.As it has a narrow therapeutic window,the dose needs to be titrated with regular monitoring of international normalized ratio (INR) (target: 2-3).Vitamin K epoxide reductase complex subunit C1 (VKORC1) and cytochrome P450 2C9 (CYP2C9) are the two major genes involved in the metabolism of warfarin and they determine the dose requirement of warfarin[39].CYP2C9 metabolizes the more potent S-enantiomer of warfarin while VKORC1 is the target protein for warfarin.Multiple single nucleotide polymorphisms have been described in these genes,of which the most important ones are CYP2C9*2,CYP2C9*3,and VKORC1 heterozygous haplotype GA and homozygous AA[40].Thus,the patient’s genotype is a major determinant of not only the dose requirement but also the risk of anticoagulationrelated complications.In a study from India in adults with BCS,patients with the presence of mutations in VKORC1 or CYP2C9 were associated with an increased risk of bleeding[41].More intensive monitoring while on warfarin is recommended for these patients.Some guidelines have now started recommending incorporating the results of genetic testing for clinical use while on warfarin therapy[42].
Identification of thrombophilia in a child has implications in terms of the need for lifelong oral anticoagulation,increased risk of other venous thromboembolic events,lifestyle modifications like avoiding oral contraceptive pills,risk of complications like leukemia in myeloproliferative disorders,and implications for other family members.In addition,there is a need to educate asymptomatic family members regarding the risk factors and lifestyle modifications.
CLINICAL FEATURES
Clinical manifestations can be diverse,ranging from acute liver failure to completely asymptomatic patients,making BCS a possible differential diagnosis in many acute and chronic liver diseases.Most patients with BCS have a chronic presentation,whereas only a small number of patients present with a fulminant type of BCS[12-14].The clinical and radiological features of children with BCS in various pediatric studies are summarized in Table 2.The usual age at presentation in children is 10 (range 1.5-17) years but BCS has been reported in children as young as 4.5 mo[11,12,32].The commonest symptom is rapidly reaccumulating ascites (83%-90%) with dilated tortuous abdominal and back veins (60%-70%)[11-14,31].Up to 15%-20% of patients can be completely asymptomatic,hence a high index of suspicion should be kept,especially when there is no clear detectable etiology for chronic liver disease and/or a prothrombotic condition exists[43].In such scenarios,proper imaging by an experienced radiologist is warranted[44].The presentation of BCS depends on the extent and rapidity of hepatic venous outflow obstruction and the development of decompressing venous collaterals.With this concept,BCS can be classified as fulminant,acute,subacute,or chronic[45,46].However,a pathological examination of the liver illustrates a dissociation between the rapidity of the clinical presentation and the acuteness of the histological damage.Up to 50% of patients clinically classified as acute have histological features of chronicity (e.g.,fibrosis or cirrhosis)[46].The prognostic value of this clinical classification in predicting mortality has not been prospectively evaluated[47].Over time,several prognostic indices have been designed to predict mortality and response to therapeutic interventions[15,18,46,47].These scoring systems incorporate clinical and laboratory features to stratify patients,although their use for the management of an individual patient is debatable[48].
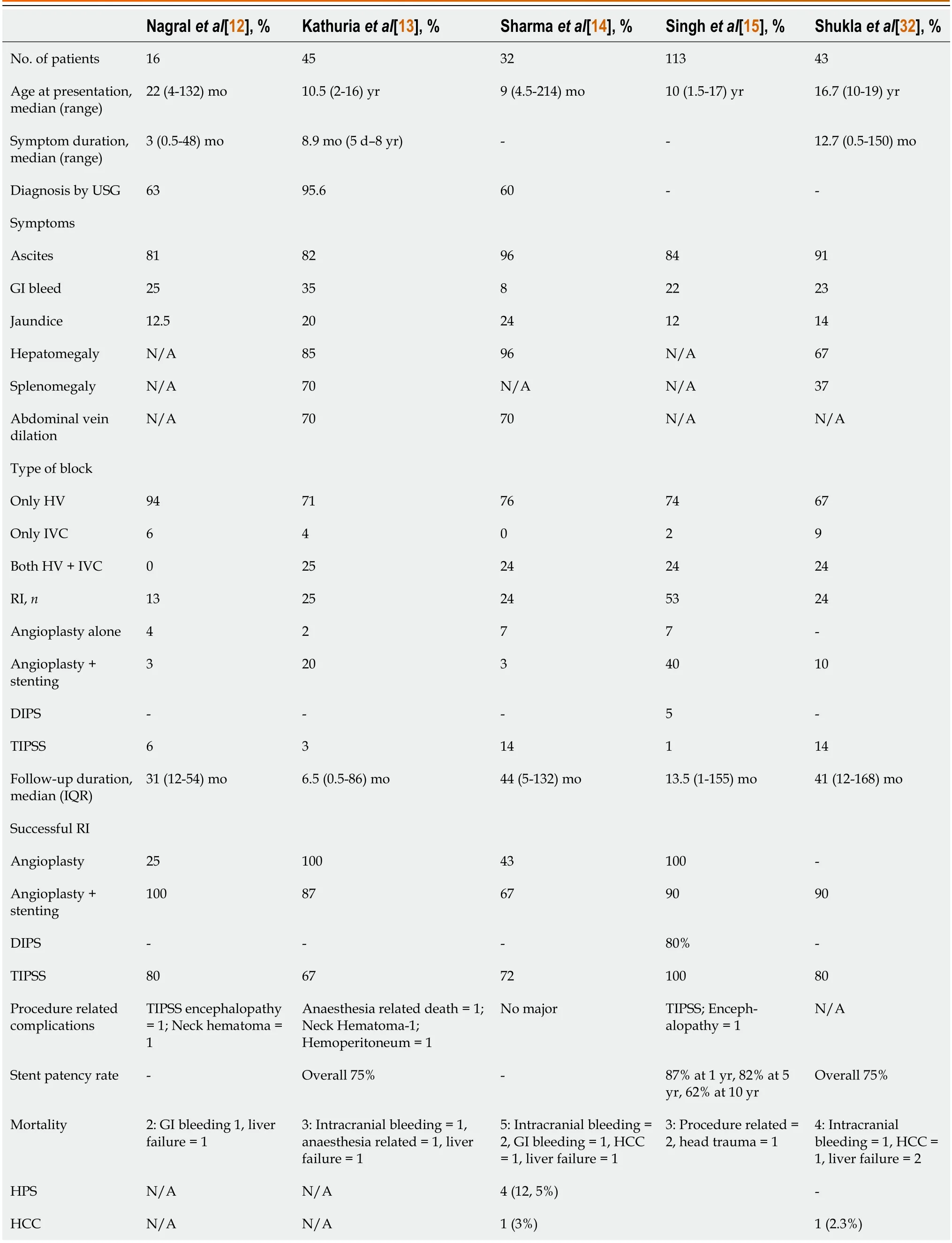
Table 2 Clinical presentation,site of block,radiological intervention,and outcomes of major studies in children with Budd-Chiari syndrome
DIFFERENCE BETWEEN ADULTS AND CHILDREN WITH BCS
BCS can occur at any age group but it most commonly affects young adults.In adults,secondary causes of BCS are much more frequently seen than children in whom primary causes are predominant.Regarding the underlying etiology,thrombophilic conditions are reported in more than 80% of adults with BCS.In Asian children,the etiology is largely idiopathic.Thrombophilia work-up is often under-reported and the results are variable.Acquired prothrombotic conditions like MPD,APLS,PNH,sickle cell disease,and oral contraceptive pill use are predominantly seen in adults.On the contrary,inherited thrombophilias like protein C deficiency and protein S deficiency are more commonly found in children.The clinical presentation is also different for adults and children.Shuklaet al[32] compared 43 children and 129 adult patients with BCS.They found hepatomegaly without ascites as the most common presentation in children as compared to ascites being the most common presentation in adults.The authors hypothesized that children have better angiogenesis and collateral formation,and shorter disease duration,leading to milder clinical presentation and better treatment outcome.
DIAGNOSTIC CHALLENGES
The diagnosis of BCS depends on the demonstration of HV and/or IVC obstruction.Invasive venography remains the gold standard;however,it is performed during the time of endovascular intervention procedure.DUS has therefore emerged as the primary imaging modality with a diagnostic accuracy of > 90%[12,13,15].DUS evaluates hepatic,portal,and IVC patency,site,and length of block and liver parenchymal changes (including caudate lobe hypertrophy and intrahepatic comma-shaped collaterals).HVs may be engorged,irregular,or filled with thrombus.The “health” and residual stump of the HV are the most critical to plan an endovascular intervention.Triphasic flow may be dampened or reversed.The IVC may be narrowed or displaced by caudate hypertrophy or contain an intraluminal thrombus.Intrahepatic comma-shaped collaterals and caudate lobe hypertrophy are almost universally seen in patients with BCS.The presence of dense intrahepatic collaterals suggests chronicity.Subcapsular collaterals may bleed during a percutaneous intervention and need to be carefully documented.Non-invasive venography (CT or MRI) is required when there is a diagnostic ambiguity.Hypoenhancement of the peripheral hepatic parenchyma and relatively normal enhancement of the caudate lobe result in a mottled appearance on CT.These changes are more obvious on MRI[49].However,if they are unyielding,invasive venography and liver biopsy should be considered.
Another challenging diagnostic dilemma in the context of BCS is hepatocellular carcinoma (HCC).Long-standing congestion and fibrosis of hepatic parenchyma in patients with chronic BCS are known to give rise to hepatic nodules.The etiology of hepatic nodules detected on radio imaging in BCS patients can be benign regenerative nodules and HCC.It is important to distinguish between these two conditions as treatment differs significantly.HCC lesions are usually larger in size (> 2 cm) and hypervascular on DUS and CT/MR[50].Also,for HCC,contrast enhancement in T1-weighted MR imaging can show different enhancement patterns between HCC with and without BCS[51].Yanget al[51],in a comparative study between 10 adult HCC patients with associated primary BCS and 32 other HCC patients without BCS,found that significantly more lesions with BCS were hyperintense during the arterial phase and slightly hyperintense or isointense during the venous phase than lesions without BCS (P< 0.05 for both).Therefore,histological confirmation is required in the workup of HCC in patients with BCS.
TREATMENT OPTIONS
Therapeutic options for BCS include medical management with anticoagulation therapy,radiological interventions such as angioplasty and stenting,surgical shunting and transjugular intrahepatic portosystemic shunt (TIPSS),and lastly,liver transplantation (LT).
Medical therapy
Anticoagulation alone with oral anticoagulants is sufficient in only 10% of adult patients,especially those with mild disease,and the vast majority of them progress,requiring intervention strategies in follow-up[52-54].Pediatric experience is limited.Sharmaet al[14] reported a 33% response in a small cohort of Indian BCS children who were treated with warfarin alone.Data on the comparative efficacy of directly acting anticoagulantsvsvitamin K antagonists in BCS patients is scarce[55].A recent Austrian multicentre study on the efficacy and safety of DOAC in 22 adult BCS patients(DOAC first-line anticoagulation in 6,switched over from warfarin and LMWH in 16) showed that DOAC showed clinical response in 63% of cases while bleeding occurred in 11 (4 major bleeding,7 minor bleeding)[55].Confirmation of efficacy and safety by larger prospective studies is needed.
Radiological endovascular intervention
The progressive improvement in radiological endovascular intervention (REI) techniques in the past two decades has provided better procedural and clinical success for BCS treatment compared to other treatment modalities in both adults and children[14,15,56,57].
Angioplasty vs stenting
The rationale of angioplasty is to decompress the liver while restoring hepatic blood flow.Angioplasty can be done with or without stenting in BCS patients with short-segment stenosis (< 5 cm).Wanget al[58],in a landmark study,compared the treatment efficacy and long-term patency of angioplasty with and without stenting in 88 adult Chinese patients with BCS.They had shown that the superiority of angioplasty with stenting over angioplasty alone in the rate of re-stenosis(2%vs40% over a median follow-up period of 27 mo,P< 0.0001)[58].Balloon angioplasty without stenting of the obstructed veins is preferred in infants and younger children as appropriate sizes of stents are not available,thus making stenting technically difficult in this population.Also due to the relatively shorter duration of disease,the veins are likely to be much more pliant.Hence angioplasty alone may reduce and normalize the venous pressure significantly after the procedure.In older children,angioplasty alone has a technical success rate and clinical response rate of 90% but is fraught with a high risk of blockage[11-15].Restenosis is almost always inevitable.Nagralet al[12],Kathuriaet al[13],and Sharmaet al[14] have reported higher re-occlusion rates with angioplasty alone (75%,33%,and 57%,respectively) compared to angioplasty+stenting (0%,13%,and 33%,respectively) and TIPSS (20%,33%,and 28%,respectively).This highlights that angioplasty with stenting rather than angioplasty alone,should be the preferred modality of radiological intervention(RI) in children.The smaller size of the liver and caliber of the veins in children pose a challenge when choosing an appropriate size and length of the stent.An uncovered self-expandable metallic stent is preferred over fully covered stents because of the lower risk of post-procedural thrombosis.Longer stents (20-30 mm) are required for long-segment IVC blocks than HV blocks.Overall,the technical success and clinical response rate of stenting in children is excellent (>90%) with a much less re-stenosis rate than angioplasty[11-15].In the authors’ experience,the length of the stent may appear longer as the liver decongests after the intervention.However,with the growth of the child,the liver also grows in size and the final stent size is usually appropriate for the child.Customized stents are available.It is also important to realize that once the liver is recovering and the physiology is restored,there is an expected sudden growth spurt in children with chronic liver disease,especially during puberty.With growth,the blood flow and turbulence through the recovering liver increase.Hence anticoagulation is most important in this phase till the stent undergoes complete endothelialization.Oral contraceptives in adolescence are to be prescribed with caution.
TIPSS
TIPSS is a shunt created between the portal and the systemic circulation,leading to a reduction in hepatic congestion and symptom resolution.Currently,TIPSS is performed in failed angioplasty-stenting with an available HV stump and as a bridge to LT[27].TIPSS is technically more demanding than cirrhotic patients due to the blocked HVs.Nonetheless,various studies have established TIPSS as a safe and effective treatment for BCS in adults[22,48,59,60].Amarapurkaret al[22] and Shalimaret al[59] have shown good technical success rates (87.5%-100%) and clinical success rates (80%-93%).Post-procedure encephalopathy occurs less frequently (3% to 5% cases) than in other cirrhotic patients,reflecting the relatively preserved hepatic function in most patients with BCS[59,60].The main concern about TIPSS is the higher occurrence of stent dysfunction.Compared to other cirrhotic patients undergoing TIPSS,BCS patients show higher stent dysfunction (70%vs50% within 1 year),probably due to underlying prothrombotic conditions in BCS[61].The advent of polytetrafluoroethylene covered stents has dramatically reduced the incidence of stent dysfunction (30%-70% in bare stentsvs10-20% in covered stents)[61,62].The available TIPSS stents are usually expensive and are inappropriate in terms of sizes for children,thus limiting their use in the pediatric population,although reports of successful treatment of children with BCS with TIPSS are available[12,13].TIPSS in children with BCS risks placement of the bare end of the stent beyond the portal vein and into the superior mesenteric vein if the size is too long.Hence,a “stent within a stent” is a minor modification of this technique that allows customization for the size of the liver.In this technique,a fully covered graft stent (10 mm × 3-7 cm) is placed inside the bare uncovered stent (10 mm × 8-10 cm).
Modified TIPSS/DIPS (direct intrahepatic portosystemic shunt)
Long-segment (> 5 cm) HV block is usually not amenable to angioplasty,stenting,or TIPSS.A modified TIPSS or DIPS is a procedure where a shunt is made between IVC and the right branch of the portal vein.Though technically challenging,DIPS has been shown to effectively decompress hepatic congestion and clinical resolution in patients with BCS.In a large pediatric study from the authors’ center,children with chronic BCS undergoing DIPS had a procedural success and clinical response rate of 80% and 90%,respectively[15].
Surgical shunts
The principle of a surgical portosystemic shunt is to relieve the obstruction causing PHTN using a venous conduit,thereby decompressing the hepatic sinusoids.Surgical portosystemic shunts have now been almost completely abandoned because of high perioperative mortality (25%) and poor shunt patency (70%)[63-65].Surgical portosystemic shunting can also be technically difficult when there is caudate lobe hypertrophy[66].Most studies on surgical portosystemic shunts in adult patients with BCS failed to show any survival benefit[65,67].In a case series of 25 Indian children with BCS,only one out of the four patients who underwent surgical shunts survived[68].
LT
LT is considered a salvage therapy in the setting of fulminant presentation,progression to end-stage liver disease,or development of HCC[27,53].BCS accounts for approximately 1% of all pediatric LT cases[69].Involvement of retrohepatic IVC,the proximity of thrombus near the right atrium,and an underlying prothrombotic condition causing vascular complications make LT challenging.The challenge is even greater when considering living donor liver transplantation(LDLT) since the graft does not contain the retrohepatic IVC,as in deceased-donor liver transplantation (DDLT).Therefore,HV reconstruction is more complex,especially if the IVC is also obliterated[69].The smaller size of vessels in children complicates the situation further.Several large retrospective analyses have evaluated the benefit of DDLT in adult BCS patients with 5-year survival rates varying between 71% and 89.4%,similar to those undergoing LT for other diseases[70,71].Due to the scarcity of deceased-donor liver grafts,LDLT has been the mainstay for BCS patients undergoing LT in most Asian countries with 5-year survival rates ranging from 75% to 81%[72-75].Data on LT in pediatric BCS is only in the form of case reports and small case series and long-term prognosis has been reported to be good so far[76,77].
STEPWISE APPROACH OR UPFRONT REI IN CHILDREN WITH BCS: THE WAY FORWARD?
The rarity of BCS in general makes it difficult to perform randomized controlled trials in patients with BCS.Hence,most recommendations regarding treatment are based on case reports,retrospective studies,and expert opinions.Concerning the timing of the interventions,the European Association for the Study of the Liver[78] and the Asia Pacific Association for the Study of the Liver (APASL)[27] recommended a stepwise therapeutic algorithm for BCS in adults.The algorithm depends on treatment response,medical therapy with anticoagulant drugs,angioplasty,stent placement,TIPS,and LT.In contrast to the strict step-up principle,an AASLD practical guideline suggested checking for a venous obstruction amenable for angioplasty in all symptomatic patients right at the beginning and treating accordingly[79].The step-up algorithm has also been criticized because it pays little attention to hemodynamics and its possible improvement or even relief by interventional treatment.The step-up algorithm also does not restore physiology at the onset and possibly delays the definitive procedure,leading to advanced liver disease.Guidelines are silent regarding what should be the optimal treatment approach in children with BCS.Longitudinal studies on pediatric BCS have shown that children with chronic BCS tend to decompensate early with ascites and variceal bleeding[13,14].In those receiving medical therapy alone,it has been seen that 26% of adults die over 2 years[53,55].Response to medical therapy has been variable in children (33-43%)and two-thirds of these children ultimately require an intervention in the long run[14].There is also concern about the safety profile of long-term use of oral anticoagulants in children[80].Hence,we suggest that all children with BCS(whether symptomatic or not) should undergo angioplasty and stenting as a primary treatment modality.
It is debatable whether asymptomatic or incidentally detected BCS should be subjected to REI or not,especially in children.On one hand,decompensation may rapidly set in as children do not tolerate portal hypertension for longer periods as compared to adults.On the other hand,there are no sound ethical justifications as to whether these children should be subjected to invasive procedures and maintained on lifelong anticoagulation.In a personal opinion,the authors would prefer the former in asymptomatic children given their longer life expectancy,milder liver disease,and possibly better health and compliance with the HV.Hence there is a small window of opportunity to restore normal physiology in optimal conditions.
POST-INTERVENTION COMPLICATIONS AND NEED FOR MONITORING AFTER REI
Immediate complications of REI include subcapsular hematoma,hemoperitoneum,congestive heart failure,transient hepatic encephalopathy,and pulmonary thromboembolism and are encountered in 1%-3% of procedures in experienced centers[12-16,27].Long-term complications of bleeding secondary to anticoagulation have also been reported[12,13,22].However,the most commonly encountered post-procedure complication is re-stenosis after REI.Singhet al[15],in a large cohort of Indian children with BCS,reported follow-up vascular patency rates of 87%,82%,and 62% at 1,5,and 10 years after intervention,respectively.In the above study,29% of the cohort with REI (27% HV/IVC stenting;60% modified TIPSS) had restenosis[15].Adult studies report 17% to 41% restenosis after REI[21,22,24].To prevent re-stenosis,heparin infusion should be started during the intervention and continued thereafter.Warfarin must be initiated within 24 h of completing the procedure.The physician should consider stopping heparin and continuation of long-term warfarin if the target INR of 2-3 is achieved.Periodic clinical examination,liver function test,and shunt patency by DUS (post-stenting:24 h,1 wk,1 mo,3 mo,and subsequently every 6 mo;post TIPS/DIPS: at 7-14 d,3 mo,6 mo,9 mo,and 12 mo) is performed.Post-intervention surveillance aims to re-intervene before critical stenosis or occlusions recur.
Assessment of response to therapy
Response to therapy implies restoration of blood flow across previously blocked HV/IVC and consequent improvement in organomegaly and liver function test (LFT),no recurrence of ascites without need of diuretics,and resolution of signs of PHTN.Kathuriaet al[13],in a study among 20 children with BCS undergoing REI,demonstrated clinical and biochemical resolution in all;however,the median follow-up duration was 6.5 mo only.Sharmaet al[14],in another study of 20 Indian children with BCS who underwent REI,showed that HV stenting or TIPS is efficacious in improving clinical features,LFT,PHTN features,and growth parameters in 66% and 72% of cases,respectively,over a median follow-up duration of 44 (range 5-132) mo.Further long-term studies need to holistically address the natural history and timelines of resolution of organomegaly,liver stiffness,varices,liver functions,growth,pubertal maturity,and quality of life in children with BCS.
LIVER AND SPLENIC STIFFNESS MEASUREMENT IN MONITORING
Recently,liver stiffness measurement (LSM) and splenic stiffness measurement (SSM) have been extensively studied as potential non-invasive markers of hepatic and splenic fibrosis and congestion and hepatic venous pressure gradient in patients with chronic liver disease[81-84].LSM and SSM can be measured by various imaging techniques [transient elastography (TE),shear-wave elastography (SWE),and MR elastography].Fraquelliet al[82],in a study of 132 patients with chronic hepatitis B and C,showed that LSM and SSM measured by TE,were reliable in predicting significant fibrosis[odds ratio (OR)=5.2 and 4.6,respectively] and cirrhosis (OR=7.8 and 9.1,respectively).SSM of < 48 kPa by TE was useful in ruling out esophageal varices.In another study by the same authors in 186 chronic liver disease patients,LSM and SSM measured by SWE were equally effective and reliable in predicting significant liver fibrosis as compared to TE,with SWE having the advantage of applicability in patients with obesity or ascites[83].The latest Baveno VII guidelines recommended that all cases of clinically advanced chronic liver disease should undergo LSM testing[85].
However,these tests are yet not standardized in children.Pediatric literature regarding LSM and SSM by elastography techniques is emerging.Chongsrisawaet al[86] reported significantly higher LSM in biliary atresia patients with esophageal varices than those without (37.72 ± 21.55vs10.97 ± 8.71 kPa,P< 0.001).Yoonet al[87] showed that an LSM value of > 18.4 kPa predicted clinically significant PH (CSPH) in children with CLD with a high sensitivity (87.5%) and specificity (84.0%).LSM has been used to monitor and assess treatment response in adult patients with BCS[88].In a study to assess short-and long-term outcomes in 32 Chinese adults with BCS undergoing REI,Wanget al[88] measured LSM using SWE at 2 d,3 mo,and 6 mo post-procedure.Mean LSM value before the procedure was 35.17 ± 10.60 kPa,which decreased to 15.36 ± 4.34 kPa and 15.68 ± 5.58 kPa at 3 mo and 6 mo post-procedure,respectively (P< 0.001).Published literature on the role of LSM in monitoring children with BCS is scarce[89].Dohareet al[89] evaluated the role of LSM in 32 children undergoing REI and showed that LSM values decreased significantly after REI.A maximal decrease is seen 24 h after REI (43.7 kPa at baselinevs22.5 kPa 24 h post-procedure,P=0.001)[75].Among the nine children developing re-stenosis after REI,re-stenosis was typically associated with an increase in LSM compared with the patient's prior measurement (median absolute increase 11.0 kPa;interquartile range [IQR] 6.1-24.4).
SSM reflects congestion as well as structural changes in the spleen as a direct consequence of the increased PHT[90,91].SSM is considered a direct and more suitable surrogate marker of PHTN and performs better for the prediction of CSPH[91,92] and esophageal varices[93,94].Suttonet al[94] evaluated SSM in 67 children with chronic liver disease and showed that SSM is a reliable predictor of CSPH at a value > 38.0 kPa [area under receiver operating curve (AUROC)=0.92,sensitivity=89%,specificity=82%,P< 0.01].Sintuseket al[95] studied 51 BA children and showed a higher SSM of 46.85(IQR 25.95-54.55) kPa in patients with varices as compared to the no-varices group [median SSM-16.54 (IQR 11.75-21.75)kPa;P< 0.001].SSM has been performed in adults with BCS (n=7) who underwent TIPSS[96].In this case series,SSM in combination with LSM may reflect the severity of the disease at presentation and the need for invasive treatment.SSM values also showed a significant decline after TIPSS over a median follow-up period of 1 year.Further studies are required to elucidate the role of LSM and SSM in monitoring BCS patients after REI.
PROGNOSTIC INDICES AND THEIR IMPLICATIONS
To date,many prognostic scores have been developed in patients with BCS to quantify the disease severity and prognosis(Table 3).The authors evaluated the prognostic accuracy of these indices in BCS children and found that pre-intervention PELD score with a cut-off of 4 (AUROC=0.809,86% sensitivity,and 75% specificity) significantly determined poor outcomes following REI.Zeitoun score independently predicted poor outcome [OR=15.4,95% confidence interval (CI):1.17-203.56,P=0.04] with a cut-off of 4.3 (AUROC=0.923,83% sensitivity,and 77% specificity) in the unintervened chronic BCS[15].Hence BCS children with a Zeitoun index > 4.3 should undergo REI without any delay.
LONG-TERM COMPLICATIONS OF BCS
Hepatopulmonary syndrome
Hepatopulmonary syndrome (HPS) occurs in a substantial portion (28%) of adult patients with BCS and balloon angioplasty can reverse HPS in patients with BCS[97].The mechanism is unknown but may be related to portal decompression.This may also explain the favorable outcomes of TIPSS creation for HPS in patients with cirrhosis and idiopathic PHTN[98].Sharmaet al[14] in a previous pediatric study reported the detection of HPS among five children in long-term follow-up,one in an un-intervened child and four after RI with patent stent (3 TIPSS,1 HV angioplasty).It is not clear as to which patients will have resolution or progress to HPS as contradicting outcomes have been noted.The possible reason for developing HPS even after reduction of portal hypertension post-RI could be increased exposure of pulmonary vasculature to vasodilator mediators like increased levels of nitric oxide,endothelin-1,tumor necrosis factor-α,and endotoxemia[98].
HCC is an uncommon but dreaded long-term complication of BCS[99,100].In a recent systematic review of adults,the prevalence of HCC in BCS is geographically varied[99].It is documented as 2.0%-46.2% in 12 Asian studies,40.0%-51.6%in two African studies,11.3% in one European study,and 11.1% in one American study[99].Irrespective of hepatitis as the underlying risk factor of HCC,the pooled prevalence of HCC was 17.6% in BCS patients (95%CI: 10.1%-26.7%),26.5%in IVC obstruction (95%CI: 14.4%-40.7%),and 4.2% in HV obstruction (95%CI: 1.6%-7.8%).When patients with HCC and concomitant hepatitis were excluded,the pooled prevalence of HCC was 15.4% (95%CI: 6.8%-26.7%).Only 3 out of the 16 included studies evaluated the risk factors for the development of HCC in BCS patients.However,there was significant heterogeneity among these studies and the results were contradicting[99].Further long-term prospective studies are necessary to evaluate risk factors for HCC in BCS patients.Data regarding the prevalence of HCC in children with BCS is limited.So far,only three cases of HCC in children with BCS have been reported[14,32,101].All of them had HCC in the 2nddecade of life,liver nodules > 3 cm,and elevated alpha-fetoprotein.One patient was on anticoagulation only[101]while the other two had blocked stents[14,32].In the authors’ own unpublished experience from the authors' center,an 18-year-old boy developed HCC 6 years after blockage of DIPS.Routine surveillance for HCC is thus warranted in BCS patients,even after undergoing REI.
FUTURE DIRECTIONS
Future studies need to elucidate the underlying thrombophilic conditions and their role in the etiopathogenesis of BCS.The use of next-generation sequencing in addition to the conventional thrombophilia work-up is warranted for better understanding and higher diagnostic yield.Further studies are needed to determine the precise role of MRA in differentiating benign liver nodules with HCC in BCS patients.Liver and splenic stiffness measurement by elastography techniques (transient elastography,shear-wave elastography,and MR elastography) may serve as a useful non-invasive marker for assessing treatment response.Despite its rarity,pediatric BCS provides a unique opportunity to study the natural history,long-term complications,and treatment outcome.
CONCLUSION
BCS is a rare but potentially treatable cause of portal hypertension with an excellent prognosis after definitive treatment.Due to recent advances in interventional radiology,radiological endovascular intervention is currently the preferred primary treatment modality.Better patient and procedure selection,choice of appropriate size and type of stent,and mandatory follow-up assessment are of utmost importance for better long-term outcomes.In future,prospective and larger studies should be undertaken to study the epidemiology and establish standardized diagnostic and therapeutic management protocols for pediatric BCS.
FOOTNOTES
Author contributions:Samanta A contributed to literature review,analysis,data collection and interpretation,and drafting of the initial manuscript;Sen Sarma M contributed to the conception and design of the manuscript,interpretation of the data,and critical revision of the initial manuscript;Yadav R contributed to interpretation of the data and the critical revision of the initial manuscript;all the authors approved the final version of the manuscript.
Conflict-of-interest statement:All the authors declare no conflict of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORCID number:Arghya Samanta 0000-0002-1768-0263;Moinak Sen Sarma 0000-0003-2015-4069;Rajanikant Yadav 0000-0002-6955-850X.
S-Editor:Liu JH
L-Editor:Wang TQ
P-Editor:Cai YX
 World Journal of Hepatology2023年11期
World Journal of Hepatology2023年11期
- World Journal of Hepatology的其它文章
- Risk of hepatitis B reactivation in patients with myeloproliferative neoplasms treated with ruxolitinib
- Function of macrophage-derived exosomes in chronic liver disease:From pathogenesis to treatment
- Global burden of cirrhosis and other chronic liver diseases due to nonalcoholic fatty liver disease,1990-2019
- Evaluation of a protocol for rifaximin discontinuation in critically ill patients with liver disease receiving broad-spectrum antibiotic therapy
- Metabolomics in chronic hepatitis C: Decoding fibrosis grading and underlying pathways
- Editorial: Metabolomics in chronic hepatitis C: Decoding fibrosis grading and underlying pathways
