Determination and Correlation of Solubilities of Four Novel Benzothiazolium Ionic Liquids with6PF?in Six Alcohols*
Determination and Correlation of Solubilities of Four Novel Benzothiazolium Ionic Liquids with6PF?in Six Alcohols*
HE Zhijian (何志堅)1,2, WANG Xiaomin (王小敏)2, YAO Tian (姚田)2, SONG Hang (宋航)2and YAO Shun (姚舜)2,**1School of Chemistry and Chemical Engineering, Mianyang Normal University, Mianyang 621000, China
2School of Chemical Engineering, Sichuan University, Chengdu 610065, China
Four novel benzothiazolium ionic liquids with6PF?([C1Bth][PF6], [C4Bth][PF6], [C5Bth][PF6] and [C6Bth][PF6]) were synthesized, and the rang of their melting points were determined between 358.35 K-424.05 K. The relationship of their melting points and the length of the straight alkyl chain on cation reflected ‘S’ type tendency. Then, the solubilities of the four ionic liquids in six lower alcohols (methanol, ethanol, 1-propanol, 2-propanol, 1-butanol and 2-methyl-1-propanol) were measured in the temperature rang of 253.15-383.15 K at atmospheric pressure with static analytical method, respectively. It was found that [C6Bth][PF6] in all investigated ionic liquids had the largest solubility in six alcohols and the solubility of [C4Bth][PF6] in methanol was very sensitive for temperature in 313.15-333.15 K, which was so-called “temperature-sensitivity”. This feature is of great significance to their application of catalyzing reaction or extraction process, and makes the recovery and reuse of ionic liquids (ILs) become easier. Moreover, the experimental solubility data were correlated with the modified Apelblat equation and λh equation, respectively. It was found that the result of correlation using two divided temperature ranges was better than that of using the whole temperature range.
ionic liquids, benzothiazolium, alkanol, solubility, temperature-sensitivity, correlation
1 INTRODUCTION
Ionic liquids (ILs), as a new class of low temperature organic molten salts composed of an organic cation and an inorganic or organic anion, can remain liquid status over a quite wide temperature range (e.g. 233.15-423.15 K) and low melting point (≤423.15 K) [1, 2]. With their various advantages such as almost non-volatility, non-flammablity, negligible vapor pressure, high thermal and electrochemical stability [3, 4] and strong structural designability [5], ILs have aroused increasing attention and being widely studied in fields like extraction [6, 7], synthesis [8] and electrochemistry [9] and so on.
Recently, many br?nsted acidic ILs were synthesized by us and other researchers [10-14]. These ILs are not conventional ILs for their higher melting points (some above 373.15 K) under atmospheric pressure and especially for the sharp change of solubility with a small-scale of temperature variation [10-12]. Thus, they can be served as homogeneous catalysts to efficiently catalyze the reactions at higher temperature and solid heterogeneous catalysts to be easily recovered by filtration at lower temperature after reaction. The same result will happen after the high-temperature extraction process with this kind of ILs as extraction auxiliary reagent. With the purpose to lay the foundation for their further applications, the solubility data of these temperature-sensitive ILs are needed to study systematically.
In this article, four kinds of benzothiazolium ILs with6PF?are synthesized as shown in Table 1. Their solubilities in six lower alcohols (methanol, ethanol, 1-propanol, 2-propanol, 1-butanol and 2-methyl-1-propanol) are measured under atmospheric pressure and correlated with a modified Apelblat equation and λh equation. These results would be beneficial to related process development and design of new ILs.
2 EXPERIMENTAL
2.1 Materials
The sources and purities of the chemicals are presented in Table 2.
2.2 Synthesis of benzothiazole ionic liquids
Four kinds of benzothiazole ionic liquids ([C1Bth][PF6], [C4Bth][PF6], [C5Bth][PF6] and [C6Bth][PF6]) were synthesized with “one-pot” method [15]. The synthesis route is shown in Fig. 1.
The synthesis steps of other three kinds of benzothiazole ionic liquids were similar to those of [C1Bth][PF6] as following:
(1) 0.05 mol (6.7590 g) benzothiazole, 0.06 mol methyl bromide and 0.06 mol six fluorine sodium phosphate were added to in 50 ml flask successively; (2) After the mixture reacted 6 h at under 100 °C, the yellow paste crude product was obtained. It was mixed with proper amount distilled water and filteredto remove unreacted salts. The filter cake was washed with 10 ml ether for three times, and then washed with distilled water until there was no precipitation formation in the washed water with the silver nitrate test. The filter cake was re-crystallized in anhydrous ethanol and colorless acicular solid [C1Bth][PF6] was finally obtained after vacuum dryness. Their purities were determined by LC-20AT HPLC (Shimadzu Corporation, Japan) with a C18silica column (3.9 mm×150 mm, 5 μm) that were all higher than 99% (by mass). Melting points of four ILs were measured by MPA100 melting point apparatus (Stanford research system, USA, resolution: 0.01 °C).
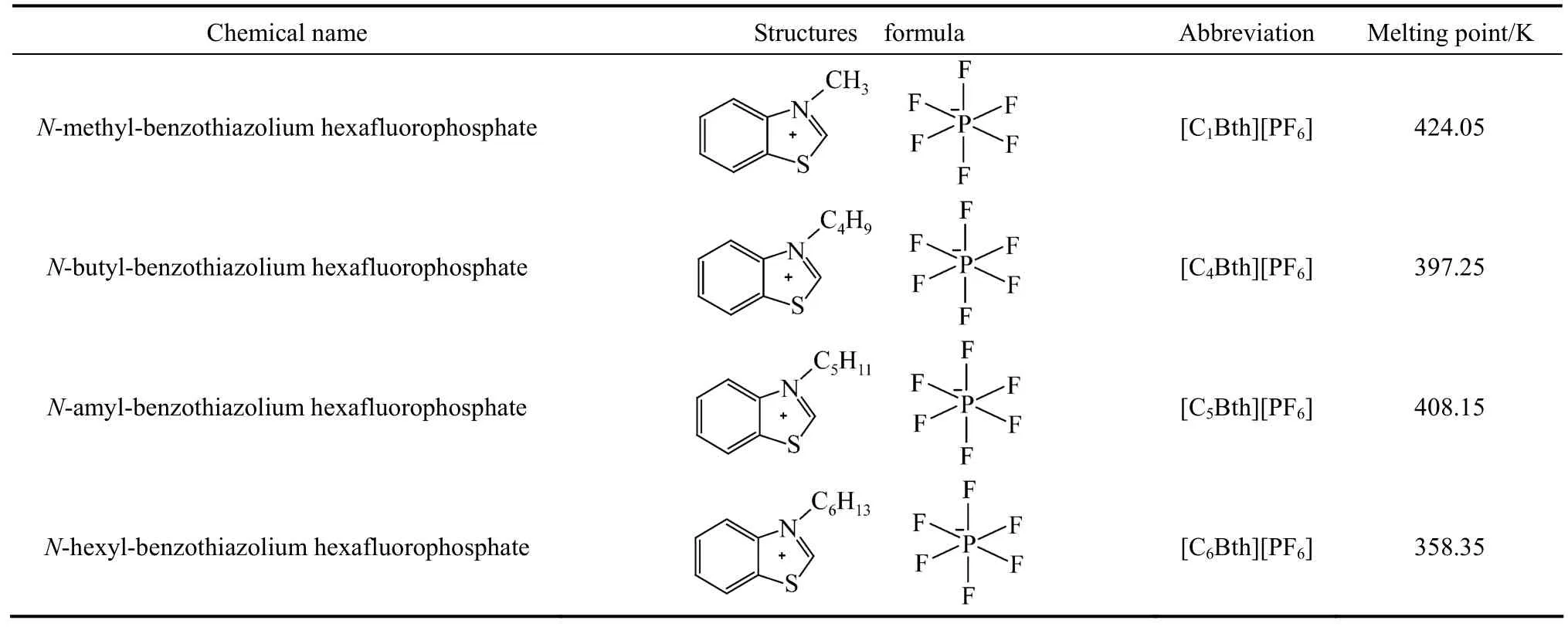
Table 1 Basic information of the four benzothiazolium ionic liquids with6PF?
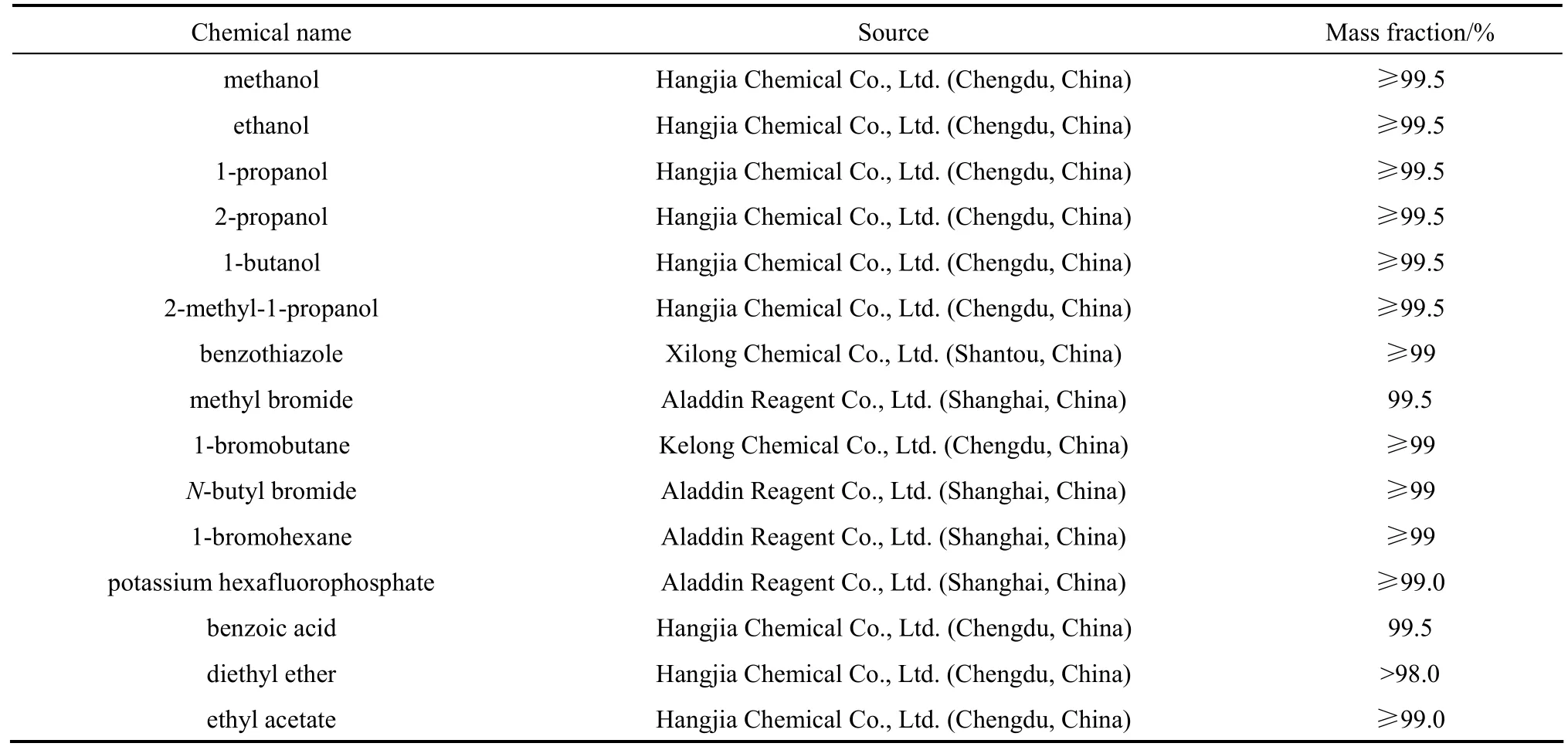
Table 2 The sources and purities of the chemicals
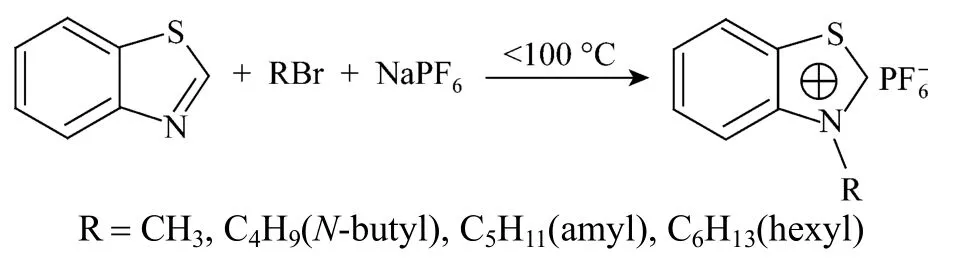
Figure 1 Synthesis route of benzothiazolium ionic liquids
2.3 Apparatus and procedure
The solubilities of ILs in alcohols were measured by
static method. Saturated solution was prepared by mixing solvent and excess IL in a three-neck flask at certain temperature with temperature stability of ±0.05 K. The temperature was controlled by constant-temperature water and oil bath pot (Yuhua Instrument Co., Ltd., Gongyi, China) or by the refrigeration cycle apparatus (Yuhua Instrument Co., Ltd., Gongyi, China) when below room temperature. The mixture was stirred for at least 2 h to reach dissolution equilibrium. After the mixture was placed for at least 1 h, about 1 ml of solution was sampled and the mass and concentration of the solution were measured by AL104 electronic balance (±0.0001 g, Mettler Toledo International, Inc., Zurich, Switzerland) and TU-1810 UV-Vis spectrophotometer (Purkinje General Instrument Co., Ltd, Beijing, China). Comprehensive standard uncertainty Uc(χ) of UV measurement was 1.12×10?3μg·ml?1and the reproducibility of concentration measurement was better than ±0.001 mass fraction. The temperature of the system was measured by a digital thermometer (±0.05K), which was directly inserted into the mixture. The mole fraction solubility (χ) was calculated with Eq. (1).
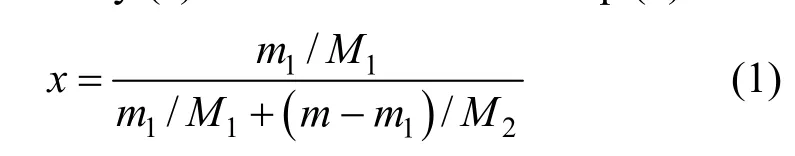
where m and m1represent the mass of solution and solute. M1and M2are the molar mass of solute and solvent, respectively. m1was calculated by the measured concentration and the volume of the diluted solution. Each measurement was repeated three times and the expanded uncertainty of the mole fraction solubility was 0.003×10?4.
To verify the measurement, the solubility of benzoic acid in 2-propanol was measured and a comparison between the experiment and the literature values [16] is shown in Fig. 2. It shows that the measured data are well consistent with the literature ones. Thus, it is proved that the measurement operation is reliable and accurate.
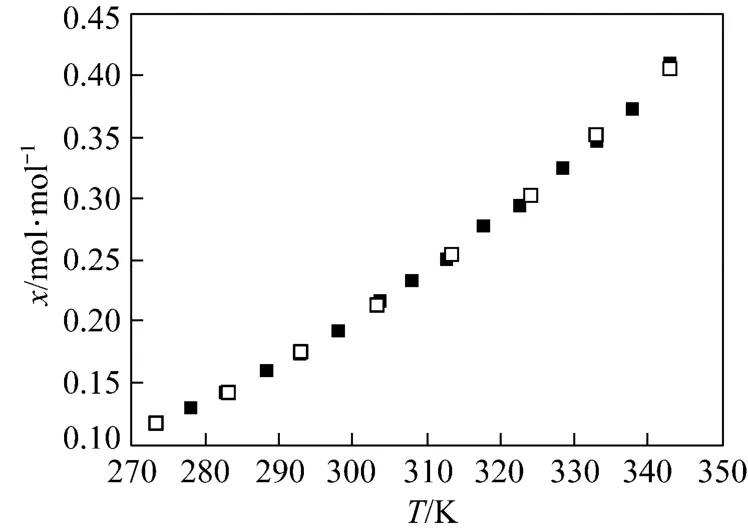
Figure 2 Comparison between the experimental and the literature values□ literature value; ■ experiment value
3 RESULTS AND DISCUSSION
3.1 Solubilities of four ILs in alcohols
The experimental values (χ) of mole fraction solubilities of [C1Bth][PF6], [C4Bth][PF6], [C5Bth][PF6] and [C6Bth][PF6] in six alcohols (methanol, ethanol, 1-propanol, 2-propanol, 1-butanol and 2-methyl-1-propanol) at 253.15-333.15 K are listed in Tables 3-6, respectively.
3.2 Effect of temperature and “temperaturesensitivity”
The solubilities (χ) of the four ILs in six alcohols increase with rising temperature and vary with different alcohols. [C6Bth][PF6] among four ILs has the largest solubility in six alcohols within whole temperature ranges. In the same alcohol, the increasing level of solubility of ILs is different in different temperature ranges. Tables 3-6 showed that the solubility of [C6Bth][PF6], [C5Bth][PF6], [C4Bth][PF6] and [C1Bth][PF6] increases 7.0, 3.0, 2.9 and 2.6 times in methanol from 253.15 K to 293.15 K, respectively. From 293.15 K to 333.15 K, they increase 6.0, 16.2, 25.8 and 8.2 times in the same condition according to the order of the foregoing, respectively. From the results, it could be found that the growth of solubility of [C6Bth][PF6] is the largest when temperature is below 293.15 K, but the growth of solubility of [C4Bth][PF6] becomes the largest in four ILs from 293.15 K to 333.15 K, which is far greater than that of other three ILs. In other words, temperature-sensitive behavior of [C4Bth][PF6] is most obvious in all of six alcohols above normal temperature, and the temperature-sensitive region (TSR) of the solubility behavior varies apparently in different alcohols (as shown in Fig. 3). This behavior or property refers to “temperature-sensitivity” of solubility and means that its solubility can be greatly changed with a small-scale variation of temperature.
The temperature-sensitive behavior makes these ILs can be easily dissolved in solution as homogeneous catalyst or extraction auxiliary reagent at high working temperature. After reaction or extraction process, they can crystallize or precipitate from the mixtures at low temperature. That means the ILs can be easily recovered by simple filtration for cycling use.
3.3 Effect of alkyl chain length of alcohols
As a result, the solubilities in the alcohols decrease along with increase of the straight chain alkyl length or carbon number of alcohol for all four ILs in 258.15-303.15 K, which is similar to that reported by previous researcher [17]. However, the variation pattern was not all valid, especially in the higher temperature range. Normally, solvent polarity will decrease with the increase of the alkyl straight chain length or carbon number of alcohol, which is not beneficial for IL dissolution. On the other hand, the increasing temperature can prompt the dissolution and the promotion could be superior to the effect of solvent polarity for these ILs when temperature is higher than 303.15 K.
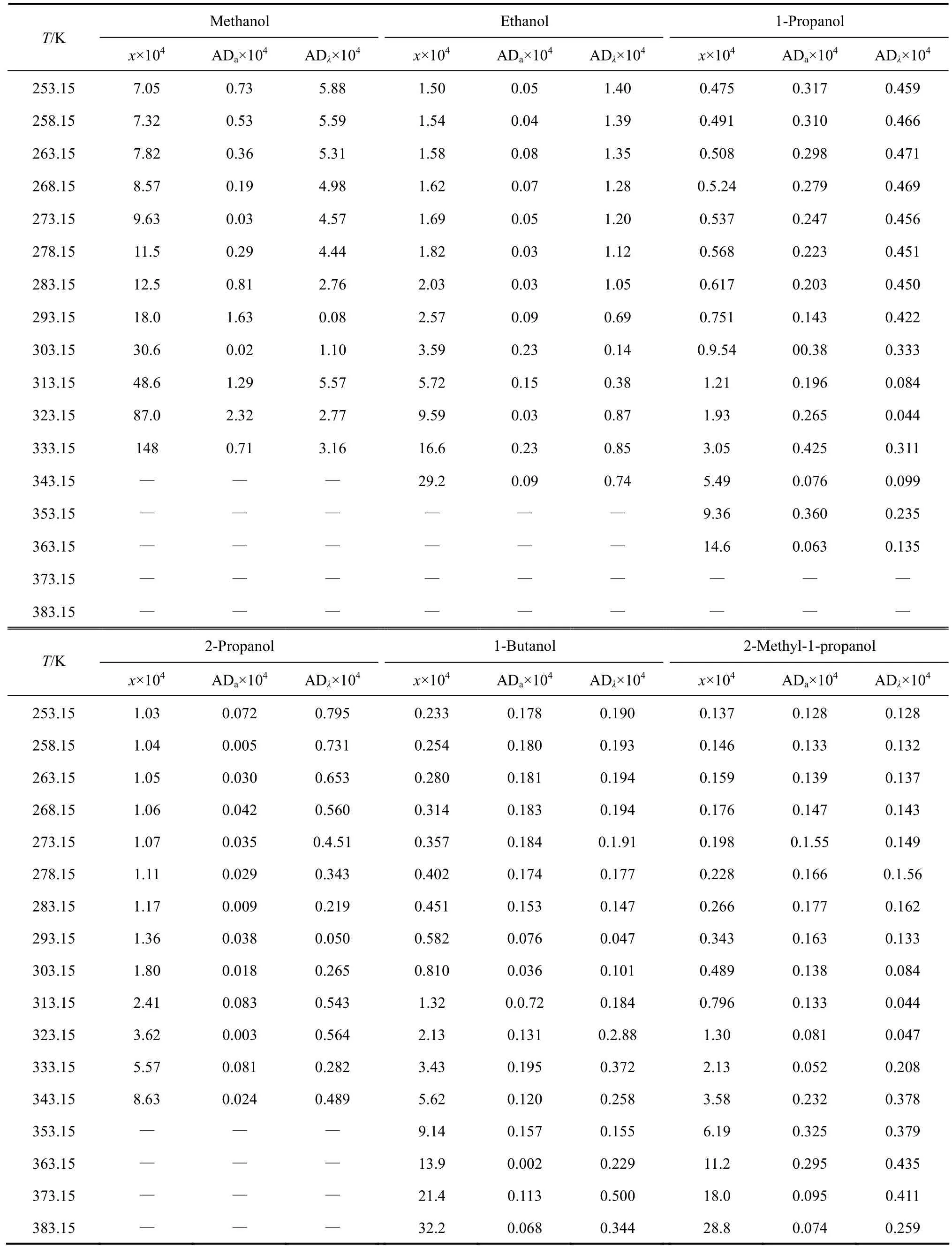
Table 3 Mole fraction solubility of [C1Bth] [PF6] in alkanols [(solid + liquid) equilibrium]
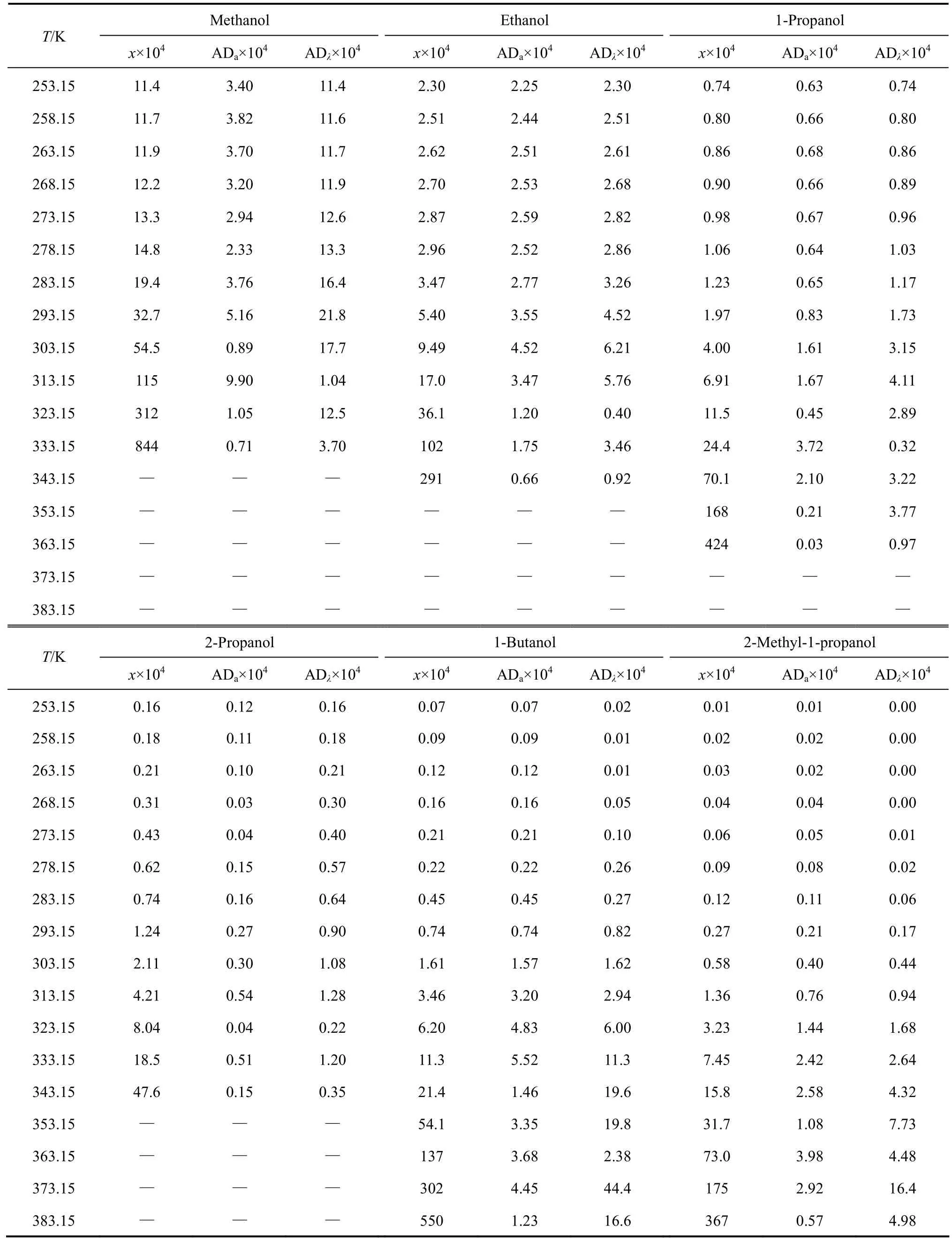
Table 4 Mole fraction solubility of [C4Bth] [PF6] in alkanols [(solid + liquid) equilibrium]
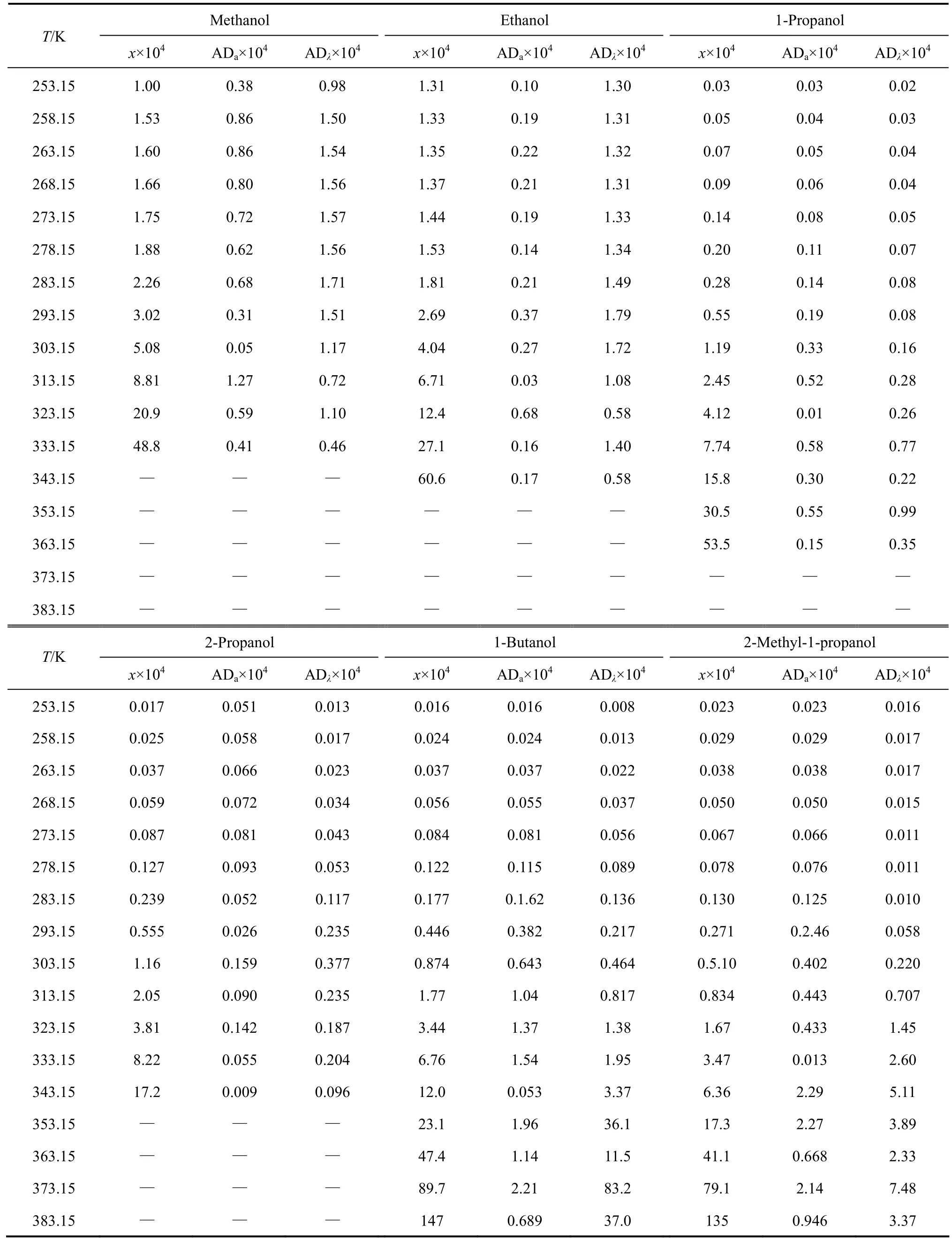
Table 5 Mole fraction solubility of [C5Bth] [PF6] in alkanols[(solid + liquid) equilibrium]
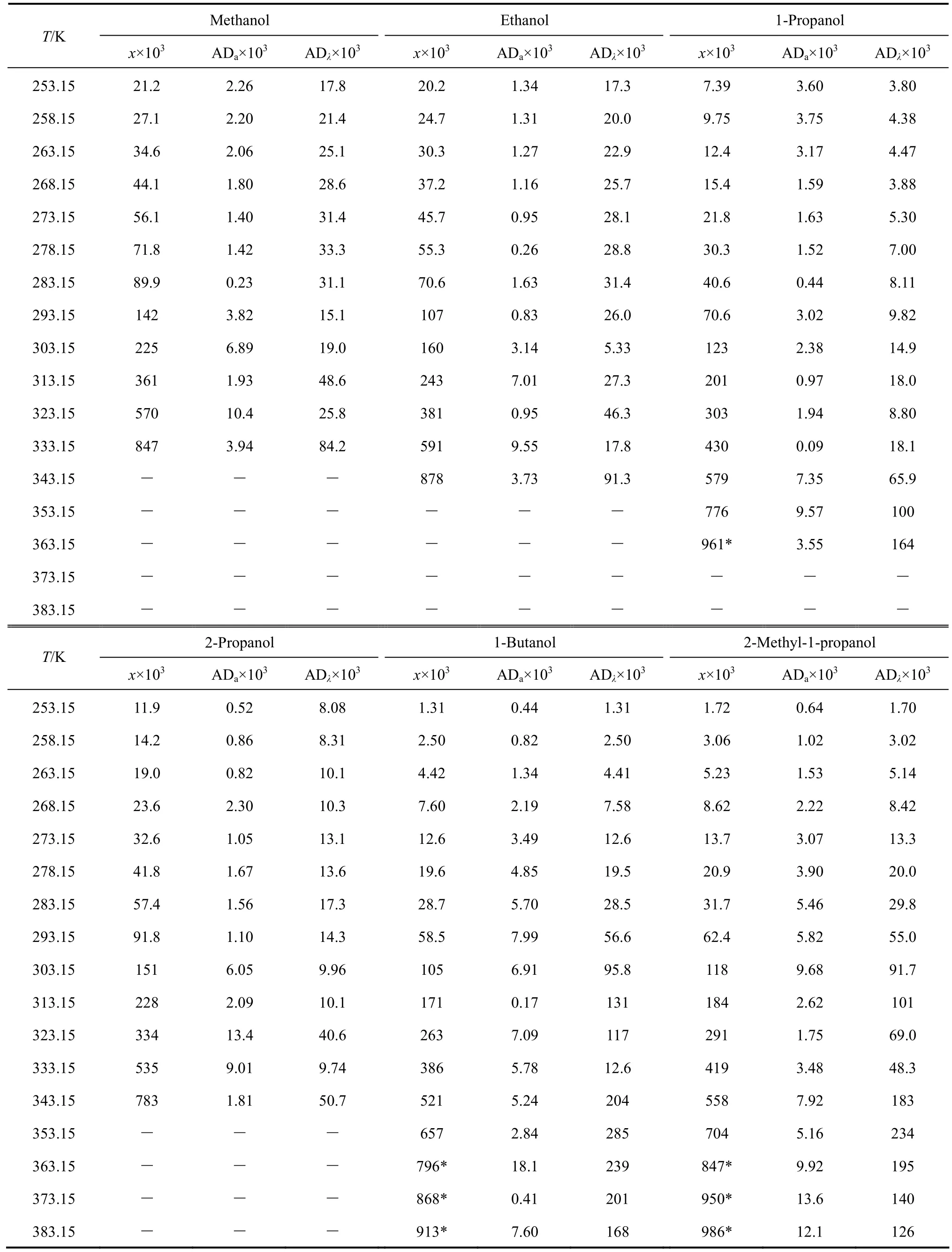
Table 6 Mole fraction solubility of [C6Bth] [PF6] in alkanols [(solid + liquid) equilibrium]
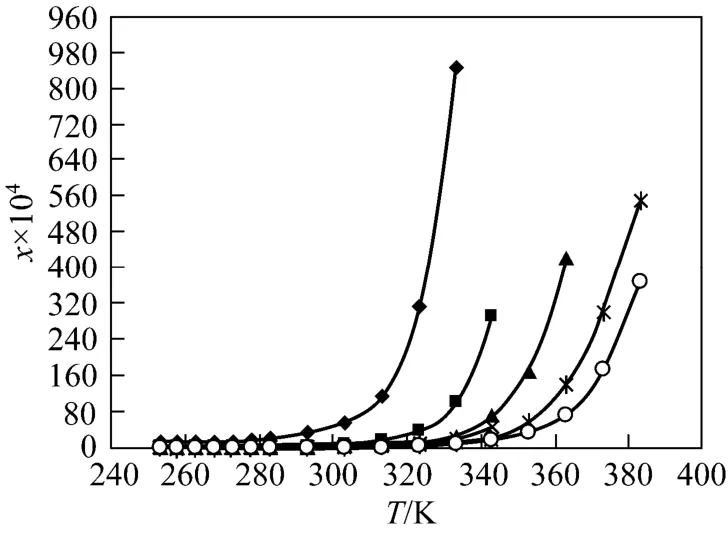
Figure 3 Solubility of [C4Bth][PF6] in six alcohols under various temperatures◆ methanol; ■ ethanol; ▲ 1-propanol;2-propanol;1-butanol;○ 2-methyl-1-propanol
3.4 Effect of the cations of ILs
The cation of ILs possesses two structural fragments including benzothiazole group area and alkyl chain area. The relationship of their melting points and the length of the straight alkyl chain on cation reflects “S” type tendency, which is similar to that of [C5Bth][PF6] reported by other researchers [18-20]. For ILs, if the volume of cation becomes larger, its structural asymmetry will be strengthened and the degree of close packing will decrease. As the result, the melting point will decrease. It is possible that when carbon atom number of straight-chain alkyl increases from C1to C4, the space stack effect of both the anions and cations will decrease with the increase of cationic space steric hindrance, and thus the melting points of ILs decline. When carbon chain is C5(N-amyl), the charged ring of thiazole is the hydrophilic region and the carbon chain is the lipophilic end, and the regions with same property will assemble and the degree of close packing will increase. Thus, the melting point of [C5Bth][PF6] rises. When carbon chain became C6(N-hexyl), volume of cationic increases, which leads to decrease of symmetry of the crystal structure. Therefore, the melting point of [C6Bth][PF6] drops.
Actually, solubility is comprehensive result of interactions between solute and solvent. Besides the effect of solvent and temperature as external cause, the structure of IL (including anion and cation) is the important internal cause, which could determine its solubility behaviors. In this case, the benzothiazolium cations of the four ILs are of different alkyls. The alkyl chain length or volume of the ILs should increase following the order of CH3, C4H9(N-butyl) and C6H13(N-hexyl). The variation tendency of solubilities of ILs seems roughly accord with that of the alkyl chain length of cation in the range of 313.15-333.15 K except [C5Bth][PF6].
3.5 Correlation of the experimental data
A variety of correlation methods of solubilities has been developed and discussed [21-25]. Supposing that the enthalpy of solution varies with temperature linearly, the experimental solubility data could be correlated as a function of temperature with one of the two non-ideal solution models. Among them the modified Apelblat equation [26] is described as
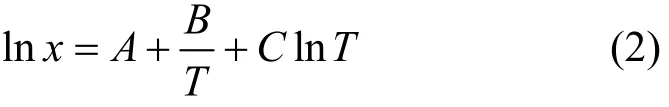
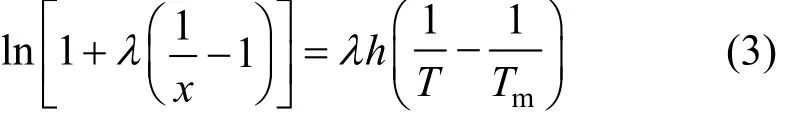
where χ is the mole fraction solubility of ILs in alcohols, T is solution temperature, Tmis fusion point of IL, and A, B, C, λ and h are correlation parameters. The absolute deviations (AD) between the experimental solubility (χi) and the calculated solubility of IL i is calculated by Eq. (4).

Moreover, the root-mean-square deviation (RMSD) is defined as Eq. (5),
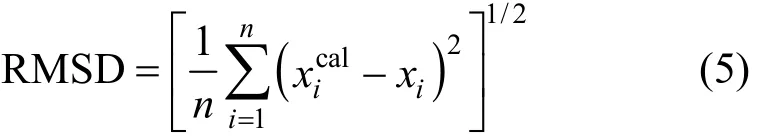
where N is the number of experimental points. The average relative deviation is defined as
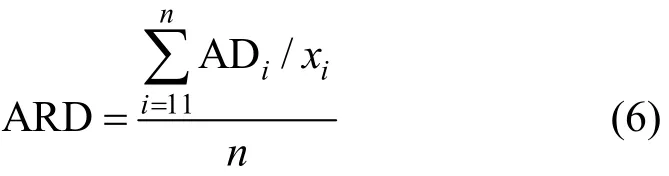
where ARD is the average relative deviation between the calculated value on different patterns and the experimental solubility, i is the ith experimental point, and n is sum total of experimental points.
ADaand ADλfor each measurement are shown in Tables 3-6. In order to directly compare the correlations, the solubility correlation is primarily performed in the same temperature period. The values of parameters, square of correlation coefficients (R2), the average absolute deviations (AAD) and the average relative deviation (ARD) together with RMSD in 253.15-333.15 K are all listed in Tables 7-8.
From the result, total average RMSD, AAD and R2are 0.001077, 0.000816, and 0.9989 for the modified Apelblat equation and 0.008515, 0.006656, and 0.9922 for the λh equation, respectively. Parameters of Apelblat equation A, B, C together with R2and ARD are listed in Table 7. Parameters of λh equation λ, h with R2and ARD are shown in Table 8.
On the whole, average value of ARD of correlation of 18 binary systems is 28.6%. The calculated values by Apelblat equation and λh equation are not in good accordance with the experimental solubility data. But correlation effect of the modified Apelblat equation is better than that of λh equation for most systems.
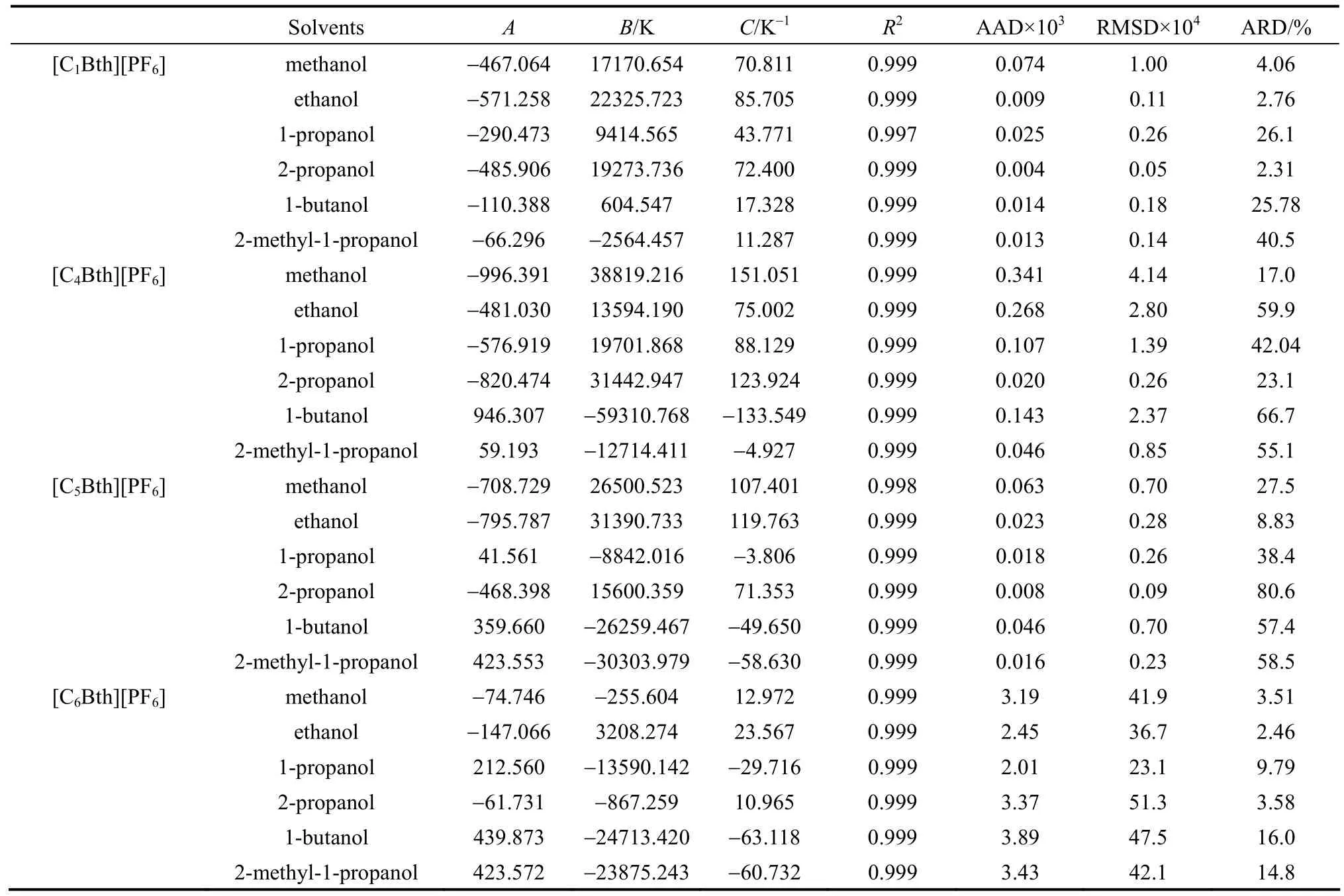
Table 7 Parameters of Apelblat equation for four ILs in alcohols in 253.15-333.15 K
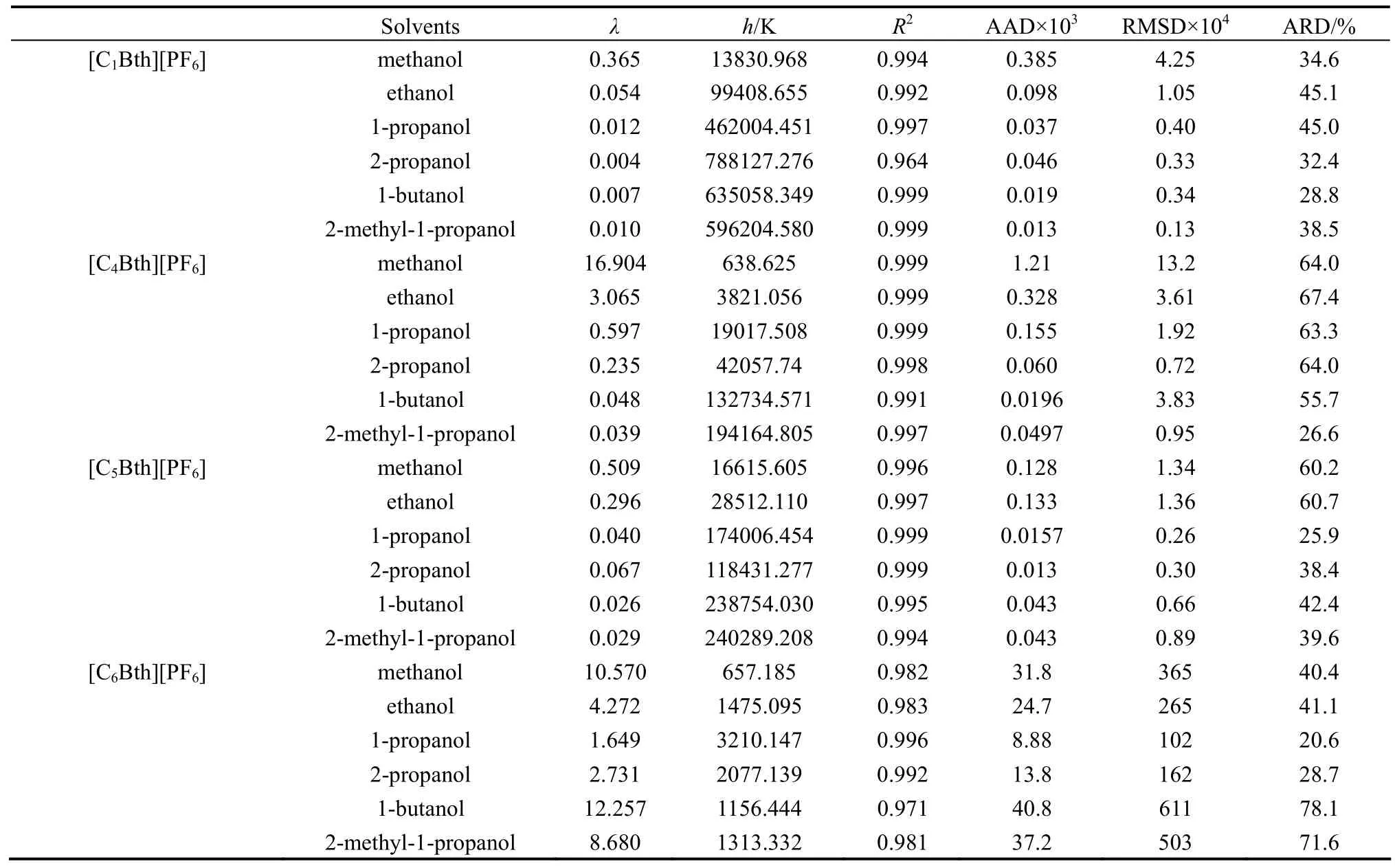
Table 8 Parameters of λh equation for four ILs in alcohols in 253.15-333.15 K
Optimization method was employed for the improvement of previous result. First, the modified Apelblat equation with the smaller ARD was selected for correlation, and then, a piecewise correlation method was employed according to the different characteristics of graphic of solubility along with temperature varying in low and high temperature range. That means the temperature range was divided into two sections in which related solubility data were correlated. Particular temperature range for the system was determined when ARD of two sections was minimal. As shown in Table 9, average value of ARD of the piecewise correlation of18 binary systems is 7.28%, decreasing 74.4% than that of the non-piecewise correlation.
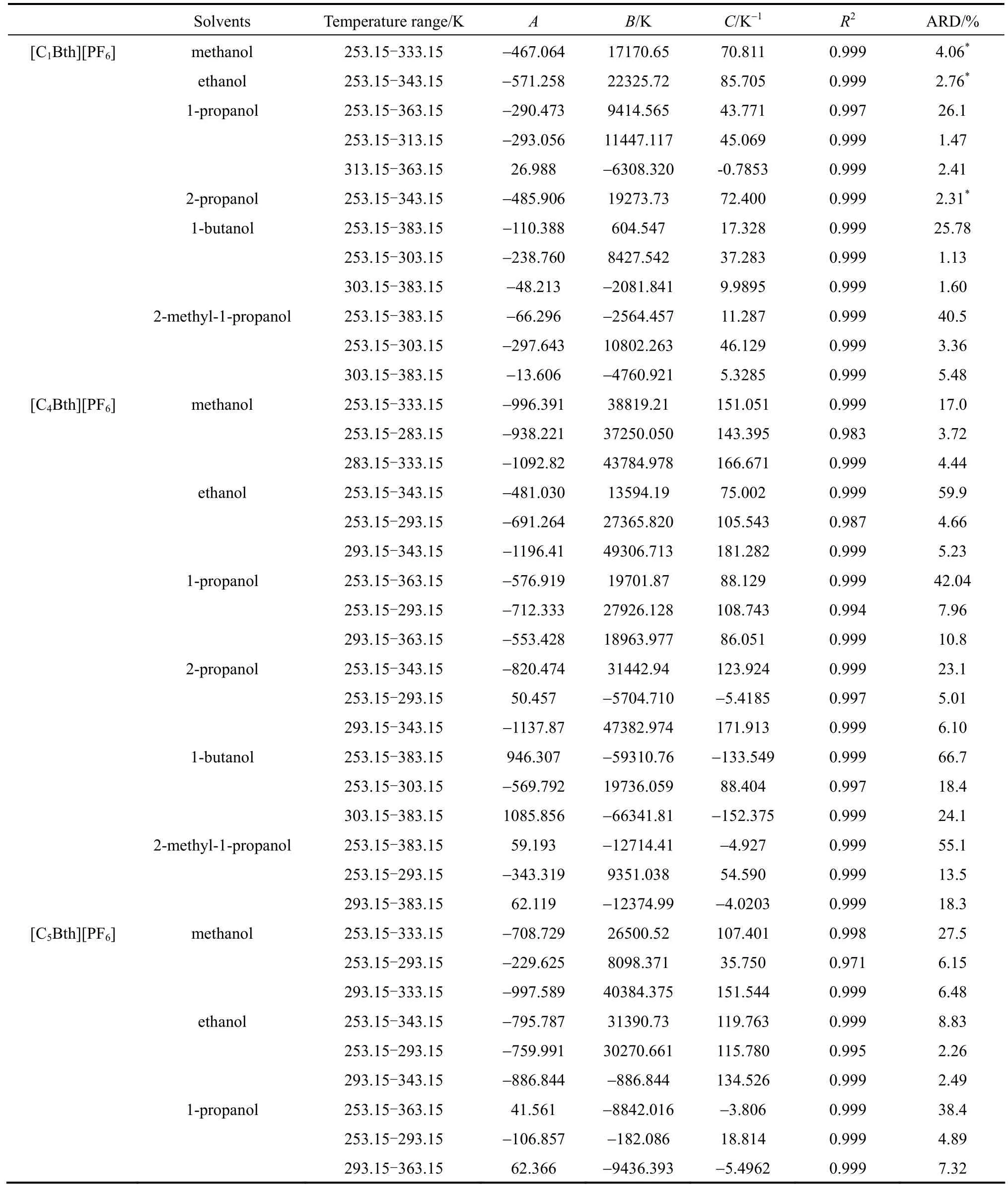
Table 9 ARD and parameters of Apelblat equation for solubility of four ILs in alcohols by the piecewise correlation
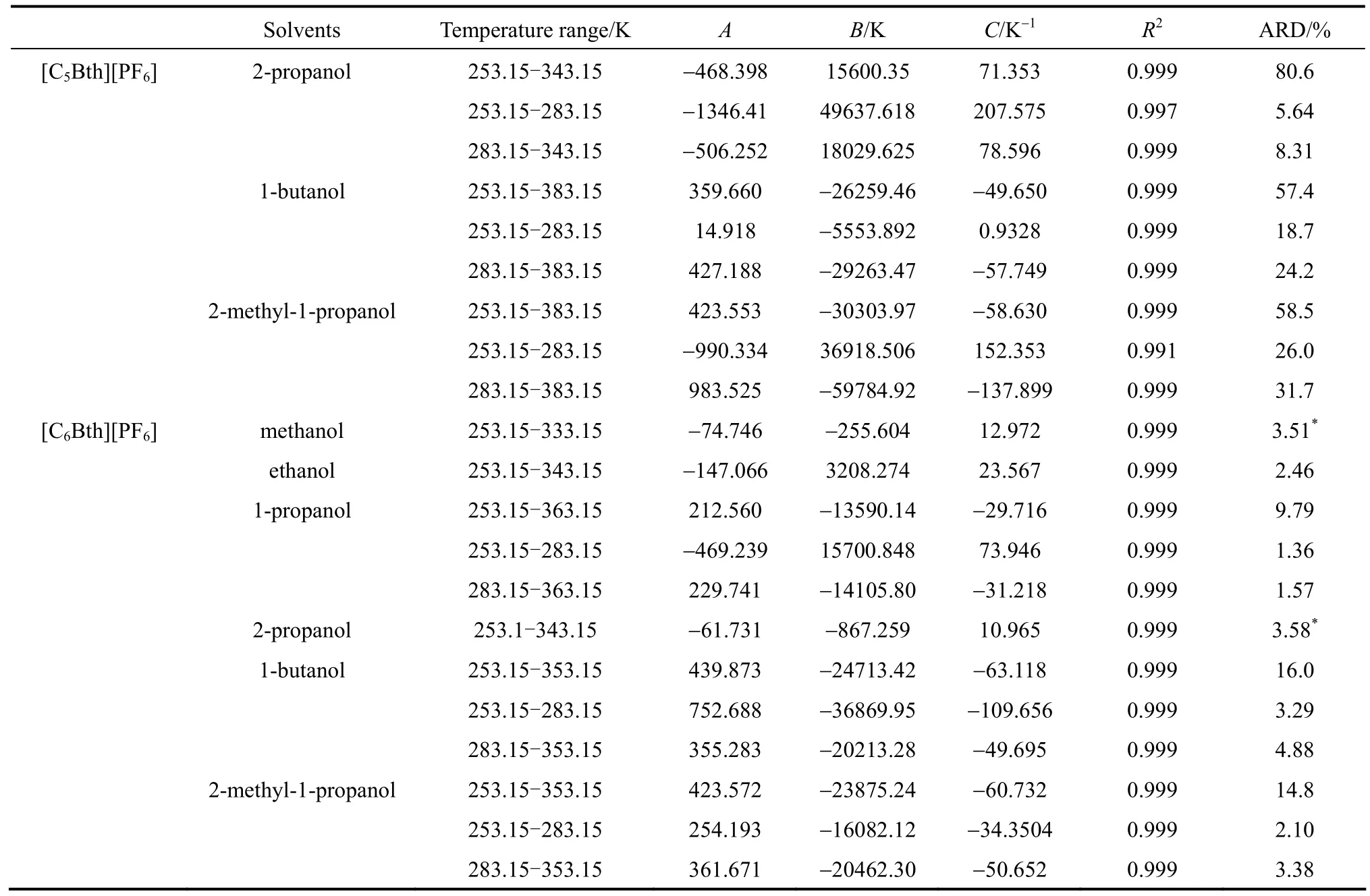
Table 9 (Continued)
4 CONCLUSIONS
The solubilities of four benzothiazolium ILs in 6 kinds of lower alcohols were measured in different temperatures under atmospheric pressure. The “temperature-sensitivity” of solubility of benzothiazolium IL could be of great significance to their easy recovery and reuse. Main variation tendency and influence factors for the solubility of these ILs have been investigated. The experimental solubility data could be correlated by the modified Apelblat equation in the piecewise correlation with acceptable accuracy. The results are expected to be helpful for related process development and design of new ILs.
NOMENCLATURE
A, B, C parameters of modified Apelblat equation
ADathe deviation between the calculated value by Eq. (2) and the measured value of solubility
ADλthe deviation between the calculated value by Eq. (3) and the measured value of solubility
h parameters of λh equation
M1, M2the molar mass of solute and solvent, respectively
N totality of experimental points
RMSD root-mean-square deviation
Tmfusion point of ILs, K
T temperature, K
U uncertainty
χiexperimental solubility of IL at ith temperature point cal i
χ the solubility of IL at ith temperature point by calculated based on the Eqs. (2) or Eq. (3)
λ parameters of λh equation
Superscript
cal calculative
Subscripts
a represent Eq. (2)
i ith temperature point
m abbreviation of fusion point
λ represent Eq. (3)
1 represent solute
2 represent solvent
REFERENCES
1 Young, G., Nippen, F., Titterbrandt, S., Cooney, M.J., “Lipid extraction from biomass using co-solvent mixtures of ionic liquids and polar covalent molecules”, J. Sep. Purif. Technol., 72 (1), 118-121 (2010).
2 Huddleston, J.G., Visse, A.E., Reiehert, W.R., Willauer, H.D., Broker, G.A., Rogers, R.D., “Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation”, J. Green Chem., 3 (4), 156-164 (2001).
3 Hu, Y.S., Wang, Z.X., Huang, X.J., Chen, L.Q., “Physical and electrochemical properties of new binary room-temperature molten salt electrolyte based on LiBETI and acetamide”, J. Solid State Ionics., 175 (14), 277-280 (2004).
4 Sun, J., Forsyth, M., Macfarlane, D.R., “Room-temperature molten salts based on the quaternary ammonium ion”, J. Phys. Chem. B., 102 (44), 8858-8864 (1998).
5 Plechkova, N.V., Seddon, K. R., “Applications of ionic liquids in the chemical industry”, J. Chem. Soc. Rev., 2008 (37), 123-150 (2008).
6 Abbott, A.P., Capper, G., Davies, D.L., Rasheed, R.K., Shikotra, P.,“Selective extraction of metals from mixed oxide matrixes using choline-based ionic liquids”, J. Inorg. Chem., 44 (19), 6497-6499 (2005).
7 De Los Ríos, A.P., Heández-Ferández, F.J., Lozano, L.J., Sánchez, S., Moreno, J.I., Godínez, C., “Removal of metal ions from aqueous solutions by extraction with ionic liquids”, J. Chem. Eng. Data., 55 (2), 605-608 (2010).
8 Parvulescu, V.I., Hardacre, C., “Catalysis in ionic liquids”, J. Chem. Rev., 107 (6), 2615-2665 (2007).
9 Abedin, S.Z.E., Endres, F., “Electrodeposition of metals and semiconductors in air and water stable ionic liquids”, J. Chem. Phys. Chem., 7 (1), 58-61 (2006).
10 Cole, A.C., Jensen, J.L., Ntai, I., Tran, K.L., Weaver, K.T., Forbes, D.C., Davis, J.H., “Novel br?nsted acidic ionic liquids and their use as dual solvent catalysts”, J. Am. Chem. Soc., 124 (21), 5962-5963 (2002).
11 Leng, Y., Wang, J., Zhu, D., Ren, X.Q., Ge, H.Q., Shen, L., “Heteropolyanion-based ionic liquids: reaction induced self separation catalysts for esterification”, Angew. Chem., 121 (1), 174-177 (2008).
12 Zhang, W.H., Leng, Y., Zhu, D.R., Wu, Y.J., Wang, J., “Phosphotungstic acid salt of triphenyl (3-sulfopropyl)phosphonium: an efficient and reusable solid catalyst for esterification”, Catal. Commun., 11 (3), 151-154 (2009).
13 Zhou, X.S., Liu, J.B., Luo, W.F., Zhang, Y.W., Song, H., “Novel br?nsted-acidic ionic liquids based on benzothiazolium cations as catalysts for esterification reactions”, J. Serb Chem. Soc., 76 (12), 1607-1615 (2011).
14 Li, X., Gu, Y.Q., Yang, Y., Song, H., Yao, S., “Novel br?nsted-acidic ionic liquids based on benzothiazolium cations as catalysts for the acetalization reactions”, J. Adv. Mater. Res., 396-398, 1969-1974 (2012).
15 Peng, Q., Zhang, Y.W., Wang, X.M., Wang, Z.D., Yao, S., Song, H.,“Synthesis of novel ionic liquid with benzothiazolium and research on their physical chemical property”, Chin. J. Synth. Chem., 20 (1), 28-31( 2012).
16 Long, B.W., Li, J., Zhang, R.R., Li, W., “Solubility of benzoic acid in acetone, 2-propanol, acetic acid and cyclohexane: Experimental measurement and thermodynamic modeling”, Fluid Phase Equilib., 297 (1), 113-120 (2010).
17 Chokradjaroen, C., Li, X.L., Tamura, K., “Mutual solubility measurements and correlations of imidazolium-based ionic liquid mixtures with alcohols”, J. Chem. Thermodyn., 46, 72-79 (2012).
18 Jiang, D., Wang, Y.Y., Liu J., Dai, L.L., “Structure-activity relationship and essential rule of the structure and melting point of imidazolium ionic liquids”, Chem., 70 (5), 371-375 (2007).
19 Tian, G.C., Li, Y.D., Jiao, Z.L., “On the structure and properties of 1-alkyl-3-methylimidazolium hexafluorophosphates [Cnmim] PF6ionic liquids”, Nonferr. Met. Sci. Eng., 1 (2), 3-9 (2010).
20 Gao, Y., Zhao, J.Y., “Discuss on the relationship between melting points of symmetric ionic liquids and molecular structure”, J. Jiangsu Inst. Edu. (Nat. Sci.), 26 (3), 9-12 (2009).
21 Shi, X.H., Li, M., Zhou, C.R., “Measurement and correlation for solubility of 2-chloro-5-chloromethyl pyridine in different solvents”, Chin. J. Chem. Eng., 18 (4), 654-658 (2010).
22 Li, Y., Lu, X.Y., “Solubilities of nizatidine in methanol + water, ethanol + water and i-propanol + water from 273.15 to 303.15 K*”, Chin. J. Chem. Eng., 20 (5), 937-941(2012).
23 Liu, Q.S., Yao, S., Zhu, T.F., Zeng, H., Song, H., “Vapor pressure measurement and Correlation of 2-methyl-butanol acetate containing calcium chloride”, Chin. J. Chem. Eng., 19 (1), 97-100 (2011).
24 Cai, S.F., Wang, L.S., Yan, G.Q., Li, Y., “Solubilities of 1-methyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate in selected solvents”, Chin. J. Chem. Eng., 18 (6), 1008-1012 (2010).
25 Lu, Y.C., Lin, Q., Luo, G.S., Dai, Q.Y., “Solubility of emodin in alcohols”, Chin. J. Chem. Eng., 17 (2), 251-253 (2009).
26 Apelblat, A., Manzurola, E., “Solubility of o-acetylsalicylic, 4-aminosalicylic, 3, 5-dinitrosalicylic, and p-toluic acid, and magnesium-DL-aspartate in water from T=(278 to 348) K”, J. Chem. Thermodyn., 31 (1), 85-91 (1999).
27 Buchowskl, H., Kslazcak, A., Pletrzyk, S., Pletrzyk, S., “Solvent activity along a saturation line and solubility of hydrogen-bonding Solids”, J. Phys. Chem., 84 (9), 975-979 (1980).
2013-08-06, accepted 2013-12-22.
* Supported by the National Natural Science Foundation of China (81102344), the Scientific Research Fund of Sichuan Province Education Department (12ZA080), Mianyang Normal University for Excellent Plan Fund (QD2012A06) and the Project of Mianyang Science and Technology Bureau (10Y003-8).
** To whom correspondence should be addressed. E-mail: cusack@scu.edu.cn
 Chinese Journal of Chemical Engineering2014年4期
Chinese Journal of Chemical Engineering2014年4期
- Chinese Journal of Chemical Engineering的其它文章
- One Step Preparation of Sulfonated Solid Catalyst and Its Effect in Esterification Reaction*
- Effects of Assistant Solvents and Mixing Intensity on the Bromination Process of Butyl Rubber*
- Determination and Correlation of Solubility for D-Xylose in Volatile Fatty Acid Solvents*
- Impacts of Power Density on Heavy Metal Release During Ultrasonic Sludge Treatment Process*
- Support Effects on Thiophene Hydrodesulfurization over Co-Mo-Ni/Al2O3and Co-Mo-Ni/TiO2-Al2O3Catalysts*
- An Approach to Formulation of FNLP with Complex Piecewise Linear Membership Functions
