Adsorption of Hg(II)from aqueous solution using thiourea functionalized chelating fiber☆
Xiaoxia Yao,Huicai Wang,2,*,Zhenhua Ma,Mingqiang Liu,Xiuqing Zhao,Dai Jia
1School of Environmental and Chemical Engineering,Tianjin Polytechnic University,Tianjin 300387,China
2State Key Laboratory of Separation Membranes and Membrane Processes,Tianjin Polytechnic University,Tianjin 300387,China
1.Introduction
With rapid industrial development,discharge of wastewater containing heavy-metal ions at high concentrations from metal plating,mining,batteries,petroleum refining,paint manufacture,photographic industries,and steel pigments increases continuously[1],which tend to accumulate in organisms and enter food chains through various pathways.Heavymetal pollutants cause serious harm to human health and are a significant threat to the environment due to their immediate toxicity and their non-biocompatibility.Mercury is one of the most toxic heavy metals commonly found in the biosphere due to its long-time,wide-range harm to human health and natural environment[2].In the European Union,the standard for drinking water quality for mercury is 1 μg·L?1,and the permitted discharge limit of wastewater for total mercury is 10 μg·L?1[3].
Therefore,it is crucial to remove Hg(II)from wastewater before discharge.Current technologies,such as ion exchange,chemical precipitation,adsorption,and membrane filtration,have been applied to eliminating toxic Hg(II)pollutants[4–6].However,some methods are limited for high operational cost and inefficiency in the removal of
☆Supported by the Tianjin and MOST Innovation Fund for Small Technology-based Firms(14ZXCXGX00724,13C26211200305)and Science and Technology Support Program(13ZCZDSF00100).
*Corresponding author.
E-mail address:wanghuicai@tjpu.edu.cn(H.C.Wang).heavy metal ions[7,8].In comparison,adsorption using low cost adsorbents is regarded as one of the most economical and effective approaches for removal of Hg(II)from aqueous solutions[9,10].A number of adsorbents have been explored for the remediation of mercury ions pollution,including activated carbon[11],carbon nanotubes[12],and natural fibers[13].Activated carbon is the most commonly used adsorbent material,but high cost usually limits its wide applications.Moreover,among the recyclable adsorption materials,those with selective adsorption for certain metal ions are scarce[14,15].
Increasing interest has been focused on the chelating fibers recently because they are effective adsorbents to remove metal ions[16–21].Chelating fibers is considered to be the most suitable adsorption material for extraction of Hg(II)from water,owing to its low mass transfer resistance,high adsorption kinetics,suitable functional groups,low cost,and relatively large external specific surface areas[22,23].Adsorption properties of chelating fibers,to a certain extent,depend on appropriate chelating groups that possess high chelating affinity toward Hg(II)ions.Thus,the adsorption efficiency,selectivity and mechanism of heavy-metal ions by chelating fibers are usually affected by the functional groups on the adsorbent surface.
It has been found that chelating functionalities such as amino and amide groups are the most effective groups for the removal of Hg(II)from water,because of their strong affinity toward mercury ions[24–26].Furthermore,Hg(II)ions have distinct affinity to bind strongly with sulfur-containing functional groups,chelating fibers have been modified with mercapto groups(?SH)to enhance the adsorption capacity toward mercury ions[27].Different adsorbents were successfully prepared[28].Wang et al.have prepared polysulfone hollow fiber affinity membrane modified with mercapto group and achieved an ideal result for removal of Hg(II)[29,30].Jainae et al.prepared a selective adsorbent with thiol-functionalized polymer for removing Hg(II)from aqueous solutions[31],which do not alter the saturation uptake value of the adsorbent while improve fiber acid resistance and adsorption efficiency.Sulfur-containing as well as amino-containing chelating agents have been attracting Significant attention for selective removal of Hg(II)from water.
Thiourea(TU)is a chelating ligand,well known for its affinity toward mercury ions owing to the presence of both amino and mercapto groups.Chelating ligands can form strong complexes with Hg(II)ions by chelation or complexation[32].Molecular structure of thiourea contains one S atom and two amino groups.S is used as ligating atom,forming coordination or complexation with Hg(II).N atoms of the amino group have lone pair electrons,which are an electron-donating group and fabricate ligand with Hg(II)easily.Moreover,amino group can produce amidation reaction with carboxyl on the surface of chelating fiber to form amide linkage[33].A standard method for immobilization of amino-containing compound into carboxyl-containing substrates via covalent amide bond is using N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride(EDC·HCl)and N-hydroxy succinimide(NHS)[34].The adsorption of TU at the mercury-aqueous solution interface has been studied,indicated by the strong directional effect of the S–Hg bond,reinforced by partial charge transfer[35].
The objective of the present work is to prepare and investigate a novel adsorption material,amide group-containing chelating fiber with high adsorption capacity and high selectivity for removal of Hg(II)from aqueous solutions.The chelating fiber is prepared by using thiourea to modify the polypropylene fiber grafted acrylic acid.Its structure,selectivity and adsorption behaviors for removal of Hg(II)are studied.The kinetics and,isotherm models are introduced to study mercury adsorption mechanism.Moreover,the effects of initial concentration,adsorption time,temperature,pH on the adsorption capacity of Hg(II)are studied.
2.Experimental
2.1.Materials and methods
All reagents were of analytical grade,and all solutions were prepared with super-pure water(treated by ion exchange and then purified by reverse osmosis).Glass wares used were repeatedly washed with HNO3and rinsed with super-pure water,according to a published procedure[36].Polypropylene fibers grafted with acrylic acid(PP-g-AA)were prepared in our laboratory.EDC·HCl(98%)and NHS(98%)were purchased from Aladdin.4-Morpholineethanesulfonic acid and phosphate buffer solution were purchased from Aladdin.Hg(II)solutions were prepared by diluting 1000 mg·L?1of stock solutions of mercury standard solution(GBS 04-1729-2004).Thiourea(TU)was purchased from Yingdaxigui Chemical Reagent Factory(Tianjin).Nitric acid(HNO3)and hydrochloric acid(HCl)were used as received.
Functional groups on the adsorbent surface were recorded with a Fourier transform infrared spectroscopy(FT-IR)(Nicolet 6700,Thermo Scientific,USA).The experiment was conducted over a spectral range of 4000–500 cm?1.
An X-ray photoelectron spectrometer(XPS)(Thermo Scientific K-Alpha equipment)was used to analyze the binding energy of sorbents for analysis of elements.
2.2.Amidation reaction of PP-g-AA
The procedures of amidation reaction of PP-g-AA fibers are as follows.The dosages of thiourea,EDC·HCl and NHS were determined by the method of titration carboxyl group content in PP-g-AA fibers.In the activation reaction,certain amounts of PP-g-AA fibers,EDC·HCl and NHS were added in 0.1 mol·L?14-morpholineethanesulfonic acid buffer solution(pH 6.0)and the reaction was conducted for 15 min with stirring at room temperature.In the coupling reaction,the activation products were removed and put in 0.1 mol·L?1phosphate buffer solution(pH 7.2),and then thiourea was added into the solution for 2 h with stirring at room temperature.The amidation fibers PP-g-AATU were washed with deionized water then with ethyl alcohol,and finally dried at 40°C under vacuum overnight.
2.3.Batch sorption experiments
Hg(II)batch adsorption experiments were performed in glass conical fl asks.0.05 g of PP-g-AA-TU fibers were added to 50 ml of Hg(II)aqueous solution.The fl asks were kept for shaking at 150 r·min?1in an orbital shaker for 2 h.The concentration of Hg(II)was determined by atomic fluorescence spectrometry.The removal rate and adsorption capability are calculated by the following equation:

where C0and Ceare the initial and equilibrium mercury ion concentrations(mg·L?1),Qeis the equilibrium adsorption capacity(mg·g?1),W is the mass of PP-g-AA-TU fibers(g),and V is the volume of solution(L).The Hg(II)uptake is considered as a function of pH,varying around 3–8,at aninitial Hg(II)concentration of 10 mg·L?1.Equilibrium is as a function of temperature(15,25,35 and 45°C).The initial Hg(II)concentration is between 0.1 and 250 mg·L?1.
2.4.Adsorption isotherm
The common models,Langmuir and Freundlich equations,were used to investigate the adsorption isotherm.Isotherm study was carried out in 50 ml of solution with initial concentrations of Hg(II)from 0.1 to 250 mg·L?1and pH of 5.0.The adsorption process was conducted with stirring for 2 h at different temperatures(15,25,35 and 45°C).
3.Results and Discussion
3.1.Characterizations

Fig.1.FT-IR spectra of PP-g-AA and PP-g-AA-TU fibers.

Fig.2.XPS patterns of PP-g-AA and PP-g-AA-TU fibers.
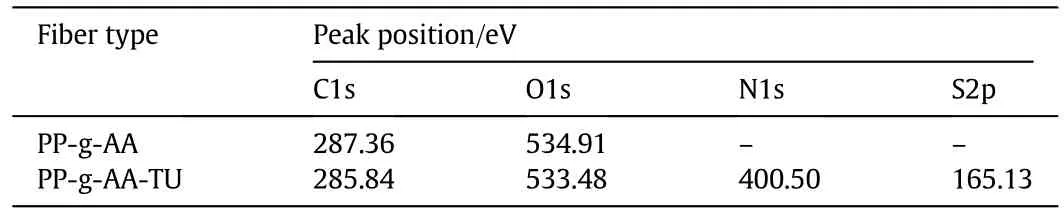
Table 1 The peak positions of C1s,O1s,N1s and S2p of PP-g-AA and PP-g-AA-TU fibers
Fig.1 shows the IR spectra of PP-g-AA and PP-g-AA-TU fibers.A broad band appears in the range of 3150–3500 cm?1in PP-g-AA-TU fibers,which should be related to stretching vibrations of–SH bonds and N–H in –NH2groups of thiourea.Compared to PP-g-AA fibers,the new peaks at 1690,1567,and 1301 cm?1might be attributed to–CONH–(C=O stretching of amide I,N–H stretching of amide II,and C–N stretching of amide III,respectively)[37].The new band at about 785 cm?1is attributed to the C–S bond.The appearance of these characteristic bands con firms that the PP-g-AA fibers are modified with thiourea successfully[38–41].
The PP-g-AA and modified chelating fibers were also examined using X-ray photoelectron spectrometry(XPS),which gives a valuable indication about the chemical changes in the modifications.The XPS survey scan of PP-g-AA and PP-g-AA-TU fibers is presented in Fig.2,and peak positions of elements are presented in Table 1.In comparison with PP-g-AA fibers,the N1s emission of PP-g-AA-TU fibers appears at 400.5 eV,indicating successful functionalization of PP-g-AA fibers with amino groups.Another peak at 165.13 eV is deduced to be S2p in the PP-g-AA-TU fibers,probably due to the insertion of sulfur-containing functional groups onto the PP-g-AA fibers.These phenomena prove that chelating fibers are successfully modified by thiourea.
Fig.3 shows the C1s XPS spectra of PP-g-AA and PP-g-AA-TU fibers.In Fig.3(a),the peakscenteringat 284.6and 285.0eVcould beassigned to C1s spectra of–C=C and CH2,respectively,while,the peaks centering at 287.9 and 289.1 eV are assigned to the C1s spectra of–C=O and–C=O–O bonding,respectively.The result suggests that,PP fiber is successfully modified by carboxylic acid.For the modified fiber(Fig.3(b)),a new peak,centering at 287.3 eV,is observed,corresponding to the C1s spectra of–C=O and–CONH.These phenomena indicate that chelating fibers are successfully modified by thiourea.
Fig.4 shows the O1s XPS spectra of PP-g-AA and PP-g-AA-TU fibers.In Fig.4(a),the peaks centering at 532.3 and 533.4 eV are assigned to O1s spectra of carbonyl and non-carbonyl of–C=O–O,respectively.The result suggests that,PP fiber is successfully modified by carboxylic acid.For the modified fiber(Fig.4(b)),a new peak,centering at 531.5 eV,is observed,corresponding to the O1s spectra of–CONH.These phenomena prove that the amid at ion reaction occurs between amino of thiourea and carboxyl in PP-g-AA-TU fiber,and the fiber is successfully modified by thiourea.
Fig.5 shows N1s XPS spectra of PP-g-AA-TU fiber.The peaks centering at 399.0 and 401.3 eV are assigned to the N1score-level spectrum of N-atoms associated with NH2or NH group bonding and the protonated imines.The appearance of these characteristic peaks con firms that the PP-g-AA fibers are modified with thiourea successfully according to the reaction equation shown in Fig.6.
3.2.Batch adsorption
3.2.1.Effect of pH on adsorption behavior
The pH value of solution was an important parameter influencing the adsorption process of metal ions. According to the calculation of stability constant, it is found that Hg(OH)2 is dominant species in the Hg(II)solution at pH > 4.0, while HgCl2 species dominates at pH < 4.0 [42].Considering the forms of Hg(II) ions at different pH values, the solution pH of 3.0–8.0 was adjusted with buffer solution.

Fig.3.C1s XPS spectra of PP-g-AA fiber(a)and PP-g-AA-TU fiber(b).
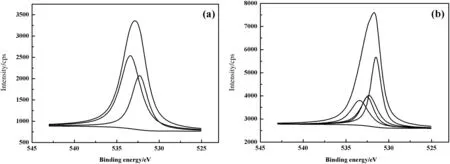
Fig.4.O1s XPS spectra of PP-g-AA fiber(a)and PP-g-AA-TU fiber(b).
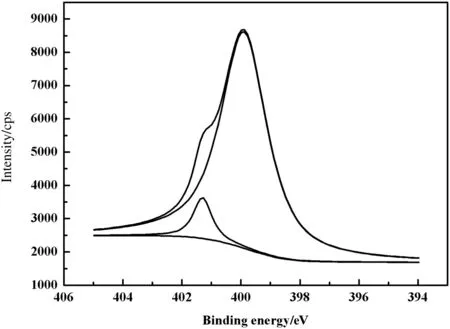
Fig.5.N1s XPS spectra of PP-g-AA-TU fiber.

Fig.6.The preparation of PP-g-AA-TU fiber.

Fig.7.Effect of pH on removal rate and the adsorption capacity of PP-g-AA-TU fibers for Hg(II)(C0=10 mg·L?1;t=2 h;T=25 °C).
Fig.7 depicts the effect of pH value on the adsorption of Hg(II)using PP-g-AA-TU fibers.pH of the aqueous solution has little influence on the adsorption,which may be due to the existence of mercapto group in adsorbents,enhancing chelating fiber acid resistance and adsorption efficiency.It is different from the result of Monier and Abdel-Latif[43],which shows that the adsorption quantity of Hg(II)increases with pH value.They considered that protons could compete with mercury ions,occupying the active sites of adsorbent below pH 3.0.Our results in this study are in good agreement with that of Balarama et al.[44].The adsorption mechanism of PP-g-AA-TU fibers may be attributed to the complex formation between mercury ions and chelating ligands,which was previously reported by Pelosi[45].
Zhang et al.has found that no Significant change of dissolved mercury at pH range of 1–12 with an initial concentration 120 mg·L?1[46],declaring that Hg(OH)2still dissolves into the solution when mercury concentration is not too high.It is evident that PP-g-AATU fibers can be effectively used as a mercury adsorbent to remove Hg(II)from aqueous solution over a wide range of pH and the most suitable value is 5,which was selected as the pH value for further experiments.
3.2.2.Effect of temperature on Hg(II)removal
The adsorption of Hg(II)as the temperature increasing from 288 to 318K is analyzed based on thermodynamic parameters such as changes in free energy(ΔG0),enthalpy(ΔH0)and entropy(ΔS0),according to the following equations:

where R(8.314 J·mol?1·K?1)is the gas constant.
With a straight line of lnKcagainst 1/T,as shown in Fig.8,the slope and intercept of the line are equal to ?ΔH0and ΔS0,respectively.The values of thermodynamic parameters are given in Table 2.The negative value of ΔG0indicates spontaneous nature of the adsorption of Hg(II)onto the adsorbent.The negative value of ΔH0is attributed to exothermic nature of adsorption process.The negative ΔH0and ΔS0values mean a chemical exothermic process accompanied by a lowering of entropy due to the adsorption of metal ions on the fiber surface,which is a common observation inmost of the metal ion uptake processes[47–55].

Fig.8.Plot of lnKcas a function of reciprocal of temperature(1/T)for the adsorption of Hg(II)by PP-g-AA-TU fibers.
3.2.3.Adsorption isotherms
Fig.9 shows the equilibrium adsorption of PP-g-AA-TU fibers in the initial Hg(II)concentration range of 0.1–250 mg·L?1.The adsorption amount of Hg(II)on the chelating fibers increases with the initial concentration until the maximum adsorption at some concentration,which may be due to a saturated state of active sites on chelating fibers.The removal rates at different concentrations remain above 97%,so the chelating fibers in this study have a Significant removal efficiency for Hg(II).The residual concentration is less than 10 μg·L?1with feed concentration of mercury below0.1mg·L?1,which meets the discharge limit of wastewater.

Fig.9.Effect of initial concentration on the adsorption capacity of PP-g-AA-TU fibers for Hg(II)(pH=5.0;t=2 h;T=25°C).
Two theoretical isotherm models,the Langmuir and Freundlich models,are often used to describe and analyze adsorption isotherms.
Equilibrium data are fitted with the Langmuir adsorption equation

where Qeis the adsorbed value of mercury ions at equilibrium concentration(mg·g?1),Qmis the maximum adsorption amount(mg·g?1),Ceis the mercury concentration(mg·L?1)in the solution at equilibrium,and b is the Langmuir binding constant related to the energy of adsorption(L·mg?1).
The experimental data are also fitted with the Freundlich isotherm

where kFand 1/nFare Freundlich constants,indicating the sorption capacity and sorption intensity,respectively.
The linear plot of Ce/Qevs.Cefor mercury adsorption is shown in Fig.10(a)and the plot of lg Qea gainstlg Ceis shown in Fig.10(b).The parameters fitted with the two models for Hg(II)with P-g-AA-TU fibers are summarized in Table 3.The Langmuir model(R2=0.9999)is much better than the Freundlich model(R2=0.9340),suggesting a monolayer adsorption.The maximum adsorption capacity obtained by Langmuir isotherm for Hg(II)is 52.08 mg·g?1,and the adsorption removal of Hg(II)is more than 97%over the initial concentration range of 0.1–250 mg·L?1.High value of the Langmuir constant indicates strong affinity of the adsorbent toward Hg(II),which may involve the sulfur containing amino acids in the chelating fiber.Mercury is characterized as a “soft”Lewis acid owing to its high polarizability.It forms strong covalent bonds with “soft”Lewis bases[56,57].
Results of Hg(II)adsorption on various adsorbents under the same experimental conditions are shown in Table 4.The maximumadsorption capacity of PP-g-AA-TU fiber for Hg(II)is higher com pared to several other chelating adsorbents.The differences of Hg(II)uptake on various adsorbents are due to their properties(function groups,surface area,etc.).

Table 2 Thermodynamic parameters for the adsorption of Hg(II)on PP-g-AA-TU fibers.

Fig.10.Linear fitting using Langmuir(a)and Freundlich(b)equations for the adsorption of Hg(II)on PP-g-AA-TU fibers.

Table 3 Fitting parameters with Langmuir and Freundlich isotherms

Table 4 Maximum adsorption capacities for the adsorption of Hg(II)onto similar adsorbents

Fig.11.Effect of contact time on the uptake of Hg(II)ions by PP-g-AA-TU fibers(C0=10 mg·L?1;T=25 °C;pH=5.0).
3.2.4.Adsorption kinetics
Fig.11 shows the adsorption kinetic curves,adsorption amount of Hg(II)on the PP-g-AA-TU fibers vs.adsorption time at the optimal pH.The adsorption of Hg(II)onto PP-g-AA-TU fibers is very rapid in the contact time from 0 to 10 min,and equilibrium adsorption quantity can be as high as 11.37 mg·L?1with 0.6 mg·g?1of the PP-g-AA fibers at the initial Hg(II)concentration of 10 mg·L?1.The phenomenon may be due to the plentiful sites and a strong ability to chelate with transition metal ions compared to carboxylic groups.These results may account for the following reasons:(1)chemical reactive sorption with a facilely instantaneous interaction between Hg(II)and reactive groups(?NH2,?CO=NH and–SH);(2)quick electrostatic interaction between Hg(II)and chelating groups;(3)chelating behavior between Hg(II)and chelating groups on the surface of chelating fibers.The metal–sulfur interaction is fundamental in the selectivity of Hg(II)by thiourea[58,59].
The uptake kinetic mechanism is explained using two common kinetic models,the pseudo- first-order and the pseudo-second-order equations,which are expressed as

where k1(min?1)is the pseudo- first-order rate constant of adsorption and k2(g·mg?1·min?1)is the pseudo-second-order rate constant,QeandQt(mg·g?1)are the amounts of metal ions adsorbed at equilibrium and time t(min),respectively.
The fitting curves of lg(Qe?Qt)vs.t and t/Qevs.t based on the experimental data are shown in Fig.12.The corresponding kinetic parameters are summarized in Table 5.It is found that the pseudo-second-order model gives higher correlation coefficient value(R2>0.9999),indicating that the pseudo-second-order equation fits the experimental data better.Therefore,this model is developed based on the assumption that the rate-determining step is chemisorption promoted by covalent forces through electron sharing between adsorbent and adsorbate.This suggests that the pseudo-second-order model is more applicable to the present adsorption kinetics and the behavior of Hg(II)sorption on PP-g-AA-TU fibers is in agreement with chemical adsorption[60,61].
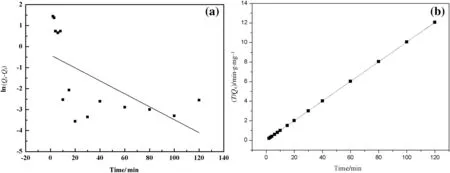
Fig.12.Fitting curve of Hg(II)adsorption kinetic model,linear fitting of pseudo- first-order(a)and linear fitting of pseudo-second-order(b).

Table 5 Kinetic parameters for the adsorption of Hg(II)
3.3.Selectivity and recyclability
Fig.13shows the selective separation ofHg2+on adsorption of PP-g-AA-TU fibers for Cd2+,Cu2+,Ca2+and Pb2+at pH 5.0(the optimum pH for Hg(II)removal)and initial concentration of 10 mg·L?1.The adsorption amount of Hg(II)by PP-g-AA-TU fibers is 9.63 mg·g?1in the presence of interference ions,so it can be used for selective adsorption of Hg(II)in aqueous solutions.
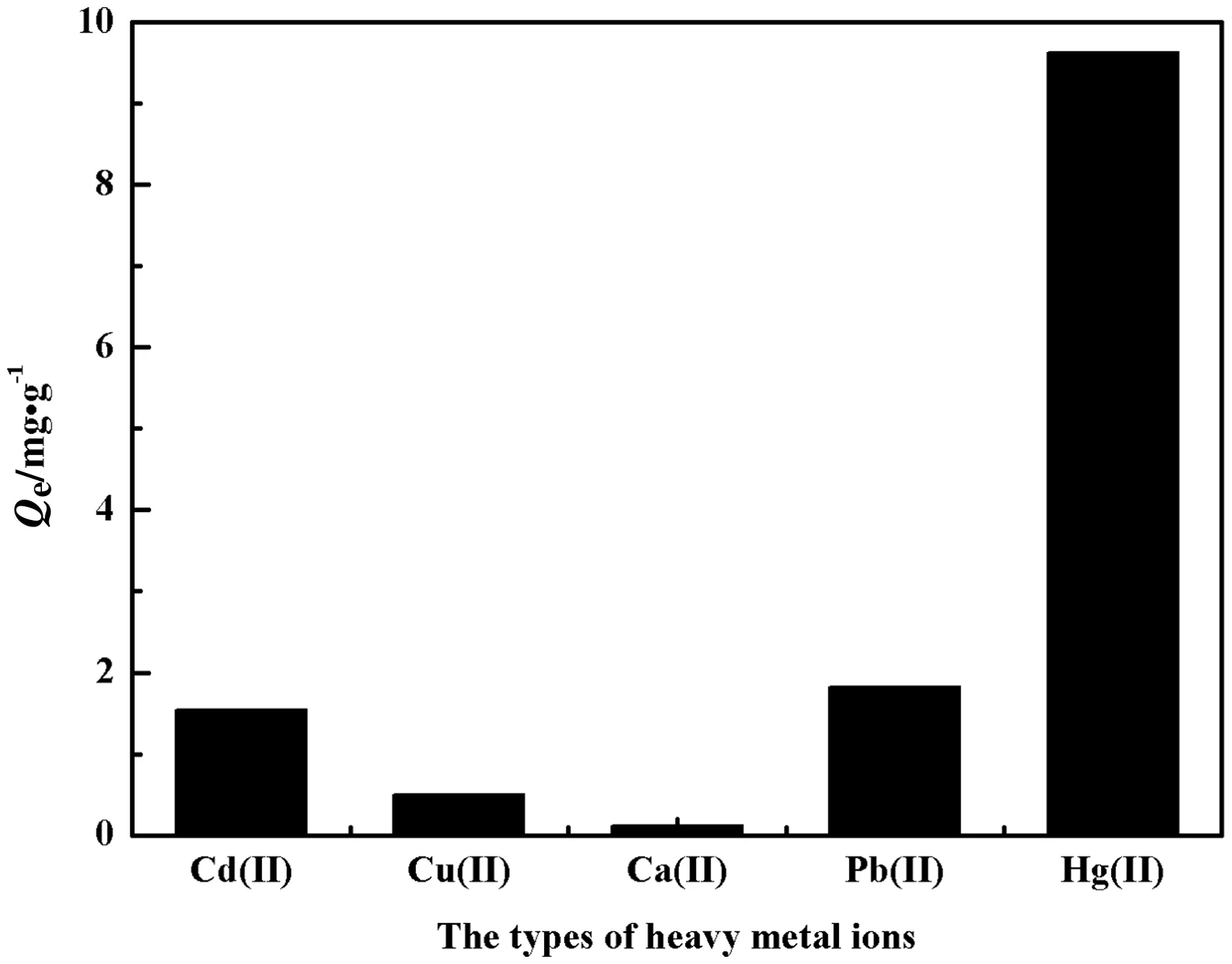
Fig.13.The adsorption capacity of interference ions by PP-g-AA-TU fibers(C0=10 mg·L?1;T=25 °C;pH 5.0).
Fig.14 shows the desorption of Hg(II)with different solutions.The desorption ratios for Hg(II)metal ions using 1 mol·L?1HCl,1 mol·L?1HNO3and 1 mol·L?1EDTA solution are 88.53%,87.32%and 81.96%,respectively.The desorption in these,solutions is mainly due to ion exchange.Adsorption capacity of PP-g-AA-TU fiber for Hg(II)can be maintained at about 80%at the 5th cycle.
3.4.Adsorption mechanism
Fig.15 shows the N1s and S2p XPS spectra of PP-g-AA-TU fiber before and after Hg(II)adsorption.As shown in Fig.15(a),399.0 and 401.3 eV could be assigned to the N1s core-level spectrum of N-atoms associated with NH2or NH groups bonding and protonated imines[62].After the adsorption of Hg(II)(Fig.15(b)),a new peak at the BE of 400.1 eV is observed and the XPS results confirm that mercury ions are adsorbed to a mine groups on the surface of PP-g-AA-TU fiber[63].The peaks suggest that the adsorbed Hg(II)ions alter the electronic properties of the bound N-atoms associated with the a mine conjugated sequences,causing the electrons to locate closer to the ions and,hence,increasing the N1s binding energy.As shown in Fig.15(c),the binding energy of the narrow XPS spectrum for S2p is 164.9 eV,corresponding to–C=S bonds.After adsorption of mercury(Fig.15(b)),the binding energy of S2p shifts to a little higher value of 166.1 eV,which may be due to the donation of the electrons from S atoms of–C=S bonds to Hg(II)[64].

Fig.14.Desorption rate of Hg(II)with different eluents.

Fig.15.N1s narrow XPS scan for PP-g-AA-TU fiber before(a)and after(b)adsorption of Hg(II);S2p narrow XPS scan for PP-g-AA-TU fiber before(c)and after(d)adsorption of Hg(II)(t=2 h;pH=5.0;T=25 °C;C0=10 mg·L?1).
Fig.16 shows schematically the proposed chelation of Hg(II)with PP-g-AA-TU fiber.Stable chelating structure forms between Hg(II),?CONH and–C=S in chelating fiber.The adsorption of Hg(II)ions onto PP-g-AA-TU fiber may be considered to consist of two processes:the first is an instantaneous adsorption stage or external surface adsorption;the second is a gradual adsorption stage with intra-particle diffusion controlling the adsorption rate until the metal uptake reaches equilibrium.

Fig.16.Proposed mode for complexation of Hg(II)with PP-g-AA-TU fiber.
4.Conclusions
A functional chelating adsorbent for Hg(II)removal from aqueous solutions is designed and prepared by using chelating molecule thiourea to modify polypropylene fiber grafted acrylic acid.The novel chelating fiber containing amide group and mercapto group can effectively remove mercury ions.PP-g-AA-TU fibers show excellent adsorption properties over a wide range of pH,and their good selectivity toward Hg(II)among some common heavy metal ions in water is due to the higher affinity of the functional groups on the adsorbent surface for Hg(II).PP-g-AA-TU fibers are superior to PP-g-AA fibers both in the equilibrium adsorption capacity and adsorption rate. The adsorption behavior suggests that the adsorption is improved by the chelation interaction between metal ions and chelating ligand.The adsorption kinetics follows the pseudo-second-order equation,and the adsorption thermodynamics indicates the exothermic and spontaneous nature of the adsorption process.The equilibrium data fit the Langmuir isotherm very well,with a monolayer adsorption capacity of 52.04 mg·g?1,and the adsorption removal rate achieves more than 97%.The results indicate great application potential of PP-g-AA-PTU fibers in water and wastewater treatment.
[1]C.G.Rocha,D.A.M.Zaia,R.V.da Silva Alfaya,A.A.da Silva Alfaya,Use of rice straw as biosorbent for removal of Cu(II),Zn(II),Cd(II)and Hg(II)ions in industrial effluents,J.Hazard.Mater.166(2009)383–388.
[2]Y.Fan,H.J.Liu,Y.Zhang,Y.Chen,Adsorption of anionic MO or cationic MB from MO/MB mixture using polyacrylonitrile fiber hydrothermally treated with hyperbranched polyethylenimine,J.Hazard.Mater.283(2015)321–328.
[3]J.A.Ritter,J.P.Bibler,Removal of mercury from waste water:Large-scale performance of an ion-exchange process,Water Sci.Technol.25(1992)165–172.
[4]Y.J.Shih,C.P.Lin,Y.H.Huang,Application of Fered-Fenton and chemical precipitation process for the treatment of electroless nickel plating wastewater,Sep.Purif.Technol.104(2013)100–105.
[5]S.Islamoglu,L.Yilmaz,H.O.Ozbelge,Development of a precipitation based separation scheme for selective removal and recovery of heavy metals from cadmium rich electroplating industry ef fl uents,Sep.Sci.Technol.41(2006)3367–3385.
[6]A.Ghaee,M.Shariaty-Niassar,J.Barzin,A.Zarghan,Adsorption of copper and nickel ions on macroporous chitosan membrane:Equilibrium study,Appl.Surf.Sci.258(2012)7732–7743.
[7]E.Salehi,S.S.Madaeni,Adsorption of humic acid onto ultra filtration membranes in the presence of protein and metal ions,Desalination 263(2010)139–145.
[8]J.W.Post,H.V.M.Hamelers,C.J.N.Buisman,In fl uence of multivalent ions on power production from mixing salt and fresh water with a reverse electrodialysis system,J.Membr.Sci.330(2009)65–72.
[9]H.Sepehrian,S.J.Ahmadi,S.Waqif-Husain,H.Faghihian,H.Alighanbari,Adsorption studies of heavy metal ions on mesoporous aluminosilicate,novel cation exchanger,J.Hazard.Mater.176(2010)252–256.
[10]F.Ma,R.Qu,C.Sun,C.Wang,C.Ji,Y.Zhang,P.Yin,Adsorption behaviors of Hg(II)on chitosan functionalized by amino-terminated hyperbranched polyamidoamine polymers,J.Hazard.Mater.172(2009)792–801.
[11]P.Girods,A.Dufour,V.Fierro,Y.Rogaume,C.Rogaume,A.Zoulalian,A.Celzard,Activated carbons prepared from wood particleboard wastes:Characterization and phenol adsorption capacities,J.Hazard.Mater.166(2009)491–501.
[12]K.Sui,Y.Li,R.Liu,Y.Zhang,X.Zhao,H.Liang,Y.Xia,Biocomposite fiber of calcium alginate/multi-walled carbon nanotubes with enhanced adsorption properties for ionic dyes,Carbohydr.Polym.90(2012)399–406.
[13]A.Ofomaja,Y.Ho,Equilibrium sorption of anionic dye from aqueous solution by palm kernel fi bre as sorbent,Dyes Pigm.74(2007)60–66.
[14]F.Zhang,Z.Zhao,R.Tan,Y.Guo,L.Cao,L.Chen,J.Li,W.Xu,Y.Yang,W.Song,Selective and effective adsorption of methyl blue by barium phosphate nanofl ake,J.Colloid Interface Sci.386(2012)277–284.
[15]A.X.Yan,S.Yao,Y.G.Li,Z.M.Zhang,Y.Lu,W.L.Chen,E.B.Wang,Incorporating polyoxometalates into a porous MOF greatly improves its selective adsorption of cationic dyes,Chem.Eur.J.20(2014)6927–6933.
[16]M.G.Hankins,T.Hayashita,S.P.Kasprzyk,R.A.Bartsch,Immobilization of crown ether carboxylic acids on silica gel and their use in column concentration of alkali metal cations from dilute aqueous solutions,Anal.Chem.68(1996)2811–2817.
[17]A.C.Templeton,F.P.Zamborini,W.P.Wuelfing,R.W.Murray,Controlled and reversible formation of nanoparticle aggregates and films using Cu2+-carboxylate chemistry,Langmuir 16(2000)6682–6688.
[18]B.L.Westcott,N.E.Gruhn,J.H.Enemark,Evaluation of molybdenum–sulfur interactions in molybdoenzyme model complexes by gas-phase photoelectron spectroscopy.The “electronic buffer”effect,J.Am.Chem.Soc.120(1998)3382–3386.
[19]J.E.McCusker,K.A.Abboud,L.McElwee-White,Carbonylation of amines with a tungsten(IV)carbonyl complex,Organometallics 16(1997)3863–3866.
[20]U.S.Schubert,C.Eschbaumer,Macromolecules containing bipyridine and terpyridine metal complexes:Towards metallosupramolecular polymers,Angew.Chem.Int.Ed.41(2002)2893–2926.
[21]B.Wrackmeyer,Metal complexes bearing terminal borylene ligands,Angew.Chem.Int.Ed.38(1999)771–772.
[22]S.B.Deng,R.B.Bai,J.P.Chen,Behaviors and mechanisms of copper adsorption on hydrolyzed polyacrylonitrile fibers,J.Colloid Interface Sci.260(2003)265–272.
[23]S.Deng,R.Bai,Removal of trivalent and hexavalent chromium with aminated poly-acrylonitrile fibers:Performance and mechanisms,Water Res.38(2004)2423–2431.
[24]H.Yoshitake,T.Yokoi,T.Tatsumi,Adsorption of chromate and arsenate by amino functionalized MCM-41 and SBA-1,Chem.Mater.14(2002)4603–4610.
[25]J.Chwastowska,E.Kosiarska,Synthesis and analytical characterization of a chelating resin loaded with dithizone,Talanta 35(1988)439–442.
[26]J.U.K.Oubagaranadin,N.Sathyamurthy,Z.V.P.Murthy,Evaluation of Fuller's earth for the adsorption of mercury from aqueous solutions:A comparative study with activated carbon,J.Hazard.Mater.142(2007)165–174.
[27]A.R.Hutchison,D.A.Atwood,Mercury pollution and remediation:The chemist's response to a global crisis,J.Chem.Crystallogr.33(2003)631–645.
[28]K.Jainae,K.Sanuwong,J.Nuangjamnong,N.Sukpirom,F.Unob,Extraction and recovery of precious metal ions in wastewater by polystyrene-coated magnetic particles functionalized with2-(3-(2-aminoethylthio)propylthio)ethanamine],Chem.Eng.J.160(2010)586–593.
[29]B.Wang,L.Huang,F.Xiao,W.Liu,X.Jiang,Preparation of a heterogeneous hollowfiber affinity membrane modified with a mercapto chelating resin,J.Appl.Polym.Sci.105(2007)1687–1699.
[30]B.Wang,B.Cheng,Y.Cui,Preparation of polysulfone hollow fiber affinity membrane modified with mercapto and its recovery properties.II.Preparation of PSF-SH hollow fiber affinity membrane for recovery of Hg,J.Appl.Polym.Sci.100(2006)4795–4803.
[31]K.Jainae,N.Sukpirom,S.Fuangswasdi,F.Unob,Adsorption of Hg(II)from aqueous solutions by thiol-functionalized polymer-coated magnetic particles,J.Ind.Eng.Chem.23(2015)273–278.
[32]A.Farrukh,A.Akram,A.Ghaffar,S.Hanif,A.Hamid,H.Duran,B.Yameen,Design of polymer-brush-grafted magnetic nanoparticles for highly efficient water remediation,ACS Appl. Mater. Interfaces 5(2013)3784–3793.
[33]A.Wolowicz,Z.Hubicki,Applicability of new acrylic,weakly basic anion exchanger purolite A-830 of very high capacity in removal of palladium(II)chloro-complexes,Ind.Eng.Chem.Res.51(2012)7223–7230.
[34]C.Wang,Q.Yan,H.B.Liu,X.H.Zhou,S.J.Xiao,Different EDC/NHS activation mechanisms between PAA and PMAA brushes and the following amidation reactions,Langmuir 27(2011)12058–12068.
[35]A.Nosal-Wiercinska,Z.Fekner,G.Dalmata,The adsorption of thiourea on the mercury from neutral and acidic solutions of perchlorates,J.Electroanal.Chem.584(2005)192–200.
[36]Y.Liu,X.Chang,D.Yang,Y.Guo,S.Meng,Highlyselective determination of inorganic mercury(II)after preconcentration with Hg(II)-imprinted diazoaminobenzene-vinylpyridine copolymers,Anal.Chim.Acta 538(2005)85–91.
[37]P.Taddei,P.Monti,G.Freddi,T.Arai,M.Tsukada,Binding of Co(II)and Cu(II)cations to chemically modi fi ed wool fi bres:An IR investigation,J.Mol.Struct.650(2003)105–113.
[38]R.Coskun,C.Soykan,A.Delibas,Study of free-radical copolymerization of itaconic acid/2-acrylamido-2-methyl-1-propanesulfonic acid and their metal chelates,Eur.Polym.J.42(2006)625–637.
[39]S.Cavus,G.Gurdag,Competitive heavy metal removal by poly(2-acrylamido-2-methyl-1-propane sulfonic acid-co-itaconic acid),Polym.Adv.Technol.19(2008)1209–1217.
[40]N.Greesh,P.C.Hartmann,V.Cloete,R.D.Sanderson,Adsorption of 2-acrylamido-2-methyl-1-propanesulfonic acid(AMPS)and related compounds onto montmorillonite clay,J.Colloid Interface Sci.319(2008)2–11.
[41]A.Kacmaz,G.Gurdag,Swelling behavior of N-t-butylacrylamide copolymer and terpolymers,Macromol.Symp.239(2006)138–151.
[42]T.Budinova,N.Petrov,J.Parra,V.Baloutzov,Use of an activated carbon from antibiotic waste for the removal of Hg(II)from aqueous solution,J.Environ.Manag.88(2008)165–172.
[43]M.Monier,D.A.Abdel-Latif,Modification and characterization of PET fibers for fast removal of Hg(II),Cu(II)and Co(II)metal ions from aqueous solutions,J.Hazard.Mater.250-251(2013)122–130.
[44]M.V.Balarama Krishna,D.Karunasagar,S.V.Rao,J.Arunachalam,Preconcentration and speciation of inorganic and methyl mercury in waters using polyaniline and gold trap-CVAAS,Talanta 68(2005)329–335.
[45]G.Pelosi,Thiosemicarbazone metal complexes:From structure to activity,Open.Cryst.J.3(2010)16–28.
[46]F.Zhang,J.O.Nriagu,H.Itoh,Mercury removal from water using activated carbons derived from organic sewage sludge,Water Res.39(2005)389–395.
[47]J.Wang,L.Xu,C.Cheng,Y.Meng,A.Li,Preparation of new chelating fiber with waste PET as adsorbent for fast removal of Cu2+and Ni2+from water:Kinetic and equilibrium adsorption studies,Chem.Eng.J.193-194(2012)31–38.
[48]M.Arslan,M.Yigitoglu,O.Sanli,H.I.Unal,Kinetics of swelling assisted grafting of 4-vinyl pyridine onto poly(ethylene terephthalate) fibers using a benzoyl peroxide initiator,Polym.Bull.51(2003)237–244.
[49]E.M.Abdel-Bary,A.A.Sarhan,H.H.Abdel-Razik,Effect of graft copolymerization of 2-hydroxyethyl methacrylate on the properties of polyester fibers and fabric,J.Appl.Polym.Sci.35(1988)439–448.
[50]M. Sacak, F.Oflaz, Benzoyl peroxide initiated graft copolymerization of poly(ethylene terephthalate) fibers with acrylic acid, J. Appl. Polym. Sci.50(1993)1909–1916.
[51]S.Mishra,P.L.Nayak,G.Sahu,Graft copolymerization of methyl methacrylate onto poly(ethylene terephthalate) fibers using thallium(III)ions as initiator,J.Appl.Polym.Sci.27(1982)3867–3875.
[52]M. Sacak, F.Oflaz, Benzoyl peroxide initiated graft copolymerization of poly(ethyleneterephthalate) fibers with acrylic acid, J. Appl. Polym. Sci(1989)539–546.
[53]K.N.Rao,M.H.Rao,H.T.Lokhande,N.R.Mody,A.G.Jog,Grafting onto polyester fibers.II.Kinetics of grafting of acrylic acid,acrylonitrile and vinyl acetate onto polyester fibers,J.Appl.Polym.Sci.23(1979)2133–2138.
[54]L.Lin,W.Gong,X.Wang,X.Li,S.Wang,Preparation and characterizations of antibacterial PET-based hollow fibers containing silver particles,Mater.Lett.65(2011)1375–1377.
[55]M.Monier,N.Nawar,D.A.Abdel-Latif,Preparation and characterization of chelating fibers based on natural wool for removal of Hg(II),Cu(II)and Co(II)metal ions from aqueous solutions,J.Hazard.Mater.184(2010)118–125.
[56]L.Zhou,Y.Wang,Z.Liu,Q.Huang,Characteristics of equilibrium,kinetics studies for adsorption of Hg(II),Cu(II),and Ni(II)ions by thiourea-modified magnetic chitosan microspheres,J.Hazard.Mater.161(2009)995–1002.
[57]M.Monier,D.M.Ayad,A.A.Sarhan,Adsorption of Cu(II),Hg(II),and Ni(II)ions by modified natural wool chelating fibers,J.Hazard.Mater.176(2010)348–355.
[58]S.L.Sun,L.Wang,A.Q.Wang,Adsorption properties of cross linked carboxymethyl-chitosan resin with Pb(II)as template ions,J.Hazard.Mater.136(2006)930–937.
[59]O.Moradi,M.Aghaieb,K.Zarea,M.Monajjemi,H.Aghaie,The study of adsorption characteristics Cu(II)and Pb(II)ions onto PHEMA and P(MMA-HEMA)surfaces from aqueous single solution,J.Hazard.Mater.170(2009)673–679.
[60]E.Guibal,C.Milot,J.M.Tobin,Metal-anion sorption by chitosan beads:Equilibrium and kinetic studies,Ind.Eng.Chem.Res.37(1998)1454–1463.
[61]P.Baskaralingam,M.Pulikesi,D.Elango,V.Ramamurthi,S.Sivanesan,Adsorption of acid dye onto organ obentonite,J.Hazard.Mater.128(2006)138–144.
[62]L.Dambies,C.Guimon,S.Yiacoumi,E.Guibal,Characterization of metal ion interactions with chitosan by X-ray photoelectron spectroscopy,Colloids Surf.A Physicochem.Eng.Asp.177(2001)203–214.
[63]N.Li,R.Bai,A novel amine-shielded surface cross-linking of chitosan hydrogel beads for enhanced metal adsorption performance,Ind.Eng.Chem.Res.44(2005)6692–6700.
[64]L.Bai,H.P.Hu,W.Fu,J.Wan,X.L.Cheng,L.Zhu,L.Xiong,Q.Y.Chen,Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions,J.Hazard.Mater.195(2011)261–275.
 Chinese Journal of Chemical Engineering2016年10期
Chinese Journal of Chemical Engineering2016年10期
- Chinese Journal of Chemical Engineering的其它文章
- CFD modeling of a headbox with injecting dilution water in a central step diffusion tube☆
- Interactions between two in-line drops rising in pure glycerin☆
- Hydrodynamics of three-phase fluidization of homogeneous ternary mixture in a conical conduit—Experimental and statistical analysis
- Nickel(II)removal from water using silica-based hybrid adsorbents:Fabrication and adsorption kinetics☆
- Reactive dividing wall column for hydrolysis of methyl acetate:Design and control☆
- Treatment of compost leachate by the combination of coagulation and membrane process☆
