Treatment of compost leachate by the combination of coagulation and membrane process☆
Zengnian Shu,Yaoping Lü,Jian Huang,2,*,Wenhui Zhang
1College of Ecology,Lishui University,Lishui 323000,China
2Institutes of Urban Environment,Chinese Academy of Sciences,Xiamen 361021,China
3Institute of Engineering and Design,Lishui University,Lishui 323000,China
1.Introduction
Composting represents one of the most cost-effective methods for treatment and final disposal of the waste from the industrial and agriculture.However,large number of leachate generated from the release of water during the decomposition of the waste.They have high concentration of dissolved biodegradable and non-biodegradable compounds[1–4].All of these pose a potential risk to nearby aquifers when there is not an adequate treatment and disposal of these liquids[5–8].The composition of the leachate is quite complex,and the treatment of leachates is expensive;therefore,several combinations of physico-chemical treatments have been applied[9–12].Biological degradation is the most common technique for leachate treatment which is based on biological mechanisms.Several technologies,such as anaerobic digester-activated sludge systems[13],anaerobic sequencing batch reactor[14]and up flow anaerobic sludge blanket[15]have been used in leachate treatment.Nevertheless,difficulties occur with the utilization of these treatments for leachate due to the non-biological degradation[16–19],and the final leachate effluent can't meet the stringent discharge regulations required.
Over the past two decades,membranes have become increasingly popular in industrial processes where reliable and repeatable purification or concentration is required[20,21].Amongst several technologies currently available for the treatment of leachate,membrane filtration has emerged as an attractive option.However,membrane filtration is not suitable as a single process in leachate treatment duo to the serious fouling.Chemical coagulation is a process used to remove suspended solids(SS),colloid particles,non-biodegradable organic compounds,and heavy metals from leachate.It is effective,simple and inexpensive and is based on the use of ferrous salts or aluminum salts that flocculate the organic components or colloid particles into bulky floccules.Complementary combination of nanofiltration with pre and post-treatment appears to be a cost-effective approach[22,23].Mariam et al.treated the land fill leachate using hybrid coagulation-nanofiltration process,and compared the performance of electrocoagulation against a conventional chemical coagulation process as a pretreatment step for the nanofiltration.The results indicated that the hybrid system can be a promising candidate for leachate treatment[24].Shrawan K.Singh et al.conducted a laboratory-scale experiment to determine the effectiveness of ozonation as a pretreatment option for treating leachate using reverse osmosis and nanofiltration membranes.An effective ozonation time of 10 min at an ozone dose of 66.7 g·m?3was selected for leachate pretreatment before membrane operation,and a maximum reduction of 78%UV-254 absorbing compounds and 23%dissolved organic carbon was observed after the leachate was treated[25].The use of hybrid coagulation–nanofiltration process might be promising in addressing the existing obstacles.However,membrane fouling is a serious problem and detailed information on the performance of hybrid process is scarce.
In the present study,leachate generated from a composting reactor in our Lab was collected, and a new combination of physicochemical and filtration processes was applied to treat this leachate. Several factors such as pH value,coagulant dosage were studied to get an optimum condition.The removal efficiency of the filtration was investigated in terms of COD,turbidity,NH3-N and TOC removals.Also,the effect of the pretreatment on the membrane fouling was investigated.The final effluent quality of leachate(COD,BOD5,SS and NH3-N)was tested to meet the Chinese land fill pollution control standard(GB16889-2008).The obtained results are expected to provide a sound understanding of the Combination of Coagulation and Membrane Process in treating composting leachate.
2.Experimental
2.1.Composting leachate and reagents
The composting leachate was collected from a cylindroid reactor in our Lab,and it's inner dimension was 1 m.A leachate sample of 15 L was collected at the reactor bottom when the composting finished.The leachate was stored in refrigerated at 4°C.Poly ferric sulfate(PSF)was used as coagulant.All chemicals used for the analytical determinations were of analytical grade.
2.2.Nanofiltration membrane
PMIA nanofiltration membrane was fabricated according to our previous report[26].Brie fl y,the casting solution with 18%of PMIA,2%of PVP,6%of LiCl and 74%of DMAC was cast on the polyester non-woven fabric and then the initial formed membrane was exposed in an oven at 120°C for 0.3 min.Finally,the nanofiltration membrane was prepared by phase inversion process in a bath with pure water.The surface and cross-section of the membranes were observed by field emission scanning electron microscopy(FESEM)(HITACHI S-4800).
2.3.Experimental set-up and protocol
2.3.1.Coagulation process
The coagulation process was conducted in a conventional six beaker bench scale jar testing apparatus.Firstly,1 L of the leachate was put in the beaker,the pH was adjusted to a fixed value,and then the desired dose of PFS was added.The samples were rapidly mixed at 200 r·min?1for 2 min,followed by 25 min of gentle agitation at a stirring speed of 25 r·min?1.The flocs were allowed to settle for 2 h and the supernatant was carefully removed by pipetting just below the surface of settled water.The total organic carbon(TOC)and turbidity of the supernatant were measured to determine the optimum pH value and PFS dosage.
2.3.2.Membrane filtration
During the membrane filtration experiment,the coagulant treated effluent was adopted as the feed solution,and the filtration was performed in a cross- flow filtration apparatus with an effective area of 20.1 cm2.Before the test,all the membranes were pressurized at 1.0 MPa with pure water for 1 h to get a stable flux,and then measured at 0.4–0.8 MPa,the permeate flux was measured by a digital balance which was connected to a computer.
2.3.3.Analytical methods
Analyses of COD,total nitrogen(TN),and total phosphate(TP)were performed based on the standard Chinese NEPA methods[27].The total organic carbon(TOC)and TN were measured using a Shimadzu TOC/TN analyzer(model TOC-VVSH).A Hach 2100 N turbidity meter was used for turbidity measurement while conductivity and pH were measured using a Metrom 781 m.
3.Results and Discussion
3.1.Leachate characteristics
The physic-chemical characteristics of leachate samples are summarized in Table 1.The leachate samples were slightly alkaline pH(>7),with a dark color,low BOD5(412 mg·L?1),and high CODCr(2780 mg·L?1),with a BOD5/CODCrratio of 0.15,which is difficult to be bio-degraded.

Table 1 Characteristics of the feed solution
3.2.Membrane characterization
The surface and cross-sectional SEM images of the fabricated nanofiltration membrane were shown in Fig.1.The membrane exhibited a smooth surface and a typical asymmetrical structure with a thin dense skin layer and sponge-like sub-layer.This structure means good rejection and high flux.During the phase inversion process,the water soluble additives(PVP,LiCl)migrated to the water and PMIA immediatelysolidified to form a membrane. As most of the solvent on the membrane surface escaped during the evaporation process,the formed membrane showed a dense surface and high rejection.The key properties of the fabricated membrane were listed in Table 2.
3.3.Coagulation process
Several factors affect the performance of the coagulation,such as the characteristics of the wastewater,coagulant used,dosage of coagulant and pH value.In this study,the pH and dosage of coagulant were optimized based on the removal of COD and turbidity.
To determine the optimum value of the initial pH,the pH of the leachate samples was varied from3to12while the amount of coagulant was fixed at 1000mg·L?1.Fig.2(a)presents the effect of pH on the COD and turbidity removal during the coagulation process.It can be seen that the COD removal efficiency increased with the pH value in the range of 1 to 6,and the highest removal percentage for COD could reach to 42.9%when the pH was 6.Then,the removal efficiency decreased to 13.9%when the pH increased to 9.As shown in Fig.2(a),the variation of turbidity removal efficiency was not Significant when the pH was lower than 9.However,it decreased from 64.2%to 46.1%when the pH increased from 9 to 12.The influence of pH on coagulation process can be explained as that H+out competes metal hydrolysis products for organic ligands when pH lower than 6,then a poor removal rate occurred.At higher pH values(pH≥6),the amount of OH?increased,and it could compete with organic compounds for metal adsorption sites,and the precipitation of metal hydroxides occurs[28].
The COD and turbidity removal efficiency was also investigated by varying the PFS dose from 300 to 1600 mg·L?1with an initial value of pH 6.As shown in Fig.2,the COD and turbidity removal increased with increasing coagulant dosages up to the optimum dosage.The removal efficiency reached to 75.3%and 62.8%for turbidity and COD removal,respectively,when the optimum dosage of PFS was 1200 mg·L?1.Then,the removal efficiency of COD and turbidity decreased as the coagulation dosage increased beyond the optimum value,probably due to the restabilization of colloidal particles in excess of coagulation[29].The similar results have been also reported by other researchers.Tatsi et al.investigated the coagulation of fresh land fill leachate using aluminum sulfate and reported a COD removal efficiency of 10%at pH 10[30].The low COD removal by chemical coagulation could be attributed to the high initial content of small organic compounds particularly organic acids in leachate.

Fig.1.The surface(a)and cross sectional(b)images of fabricated membranes.

Table 2 Key performances of the membrane used in this study

Fig.2.Turbidity and COD removal as a function of pH(a)and coagulant dosage(b).
3.4.Membrane filtration
3.4.1.Effect of operation pressure and temperature
The effect of operation pressure and temperature on the separation performance on the leachate was evaluated in terms of permeate flux and TOC removal.Fig.3(a)shows that as the operation pressure increased from 0.4 MPa to 0.8 MPa,the permeate flux enhanced from 53.2 L·m?2·h?1to 102.1 L·m?2·h?1,while the TOC removal was relatively stable.As the operation pressure increased,the driving force for solutions pass through the membrane increased accordingly.So the velocity of the water to permeate through the membrane increased,and resulted in higher flux.
Fig.3(b)shows that,as the operation temperature increased from 25 °C to 65 °C,the permeate flux increased Significantly from 53.2 L·m?2·h?1to 75.1 L·m?2·h?1,the removal ratio for TOC showed a minor decline from87.8%to85.1%.The obvious enhancement of flux is mainly due to the increase in the movement of water molecules and the decrease in the viscosity of the solution[31].The constant rejection for organic matter at higher temperature partly demonstrated the thermal stability of the membrane.
3.4.2.Membrane separation performance during long-term operation
The PMIA nanofiltration membrane performance in the long-term purification process for leachate was conducted with coagulant pretreated leachate at 0.6 MPa at 25°C.The membrane was washed with pure water after every 24 h operation.Fig.4 shows the water flux evolution of the nanofiltration membrane for 5 rounds during the 120 h operation.Apparent flux decline during each run could be observed,mean that serious fouling occurred in the long term of nanofiltration.This is due to the increase of the solution viscosity and the adsorption of the organic matter on the membrane surface,due to the increase of solution concentration as the experiment went on.At last,the end flux of the last round was 50.8 L·m?2·h?1which decreased about 36.6%comparing to the initial flux of the first round.It is evident that irreversible fouling is gradually developed during the long-term filtration,indicating that chemical cleaning should be periodically carried out in order to sustain the operation.

Fig.3.Effect of operating pressure(a)and temperature(b)on the separation performances of membrane.
Fig.5 shows the average removal efficiency of the pollutants for the nanofiltration membrane.About 89.7%of COD,78.2%of TOC,72.5%of TN,83.2%of TP,and 78.6%of NH3-N were retained by the membrane.Also,the rejection of Ca2+and Mg2+was very high,could reach to 89.6%and 90.3%,respectively.The overall performances of the combined treatment processes operated under the optimum conditions are listed in Table 3.The concentration of COD,NH3-N,TOC,and SS was 92 mg·L?1,21 mg·L?1,73 mg·L?1and 23 mg·L?1,respectively.The effluent concentration reached the local discharge standard(GB16889-2008)(COD ≤100 mg·L?1,SS ≤ 30 mg·L?1and NH3-N ≤25mg·L?1).These results indicated that the combination of coagulation and nanofiltration process was an effective method to treat the composting leachate.

Fig.4.Water flux during the long-term filtration experiment for the leachate.

Fig.5.Removal of pollutants during the long-term filtration experiment.
3.4.3.Effect of pre-treatment on the membrane fouling
Membrane fouling is a serious problem in the leachate filtration process;it is associated with the deposition of the organic matter,colloidal particles and inorganic salts on the membrane surface or within the pore structure[32].To investigate the role of coagulation pretreatment in membrane fouling control,the fouling resistant was calculated according to the reference.Here,at first,water flux of the membrane was measured(Jw1)according to Eq.(1),and then the feed solution reservoir was refilled with a raw composting leachate or pretreated leachate,followed by calculation of the flux(Jc).After 2 h of filtration process,the membrane was washed with de-ionized water for 30 min and the permeate water flux of the cleaned membranes was remeasured(Jw2).Fouling caused by reversible resistances(Rr)and irreversible resistances(Rir)is calculated by Eqs.(1)and(2),the flux recovery ratio(FRR)was also calculated[33].

Previous studies have established that chemical coagulation could remove organic matter and colloidal particles effectively,it should alleviate the membrane fouling remarkably.Fig.6 shows the FRR,Rrand Rirof membranes for raw composting leachate and pretreated compostingleachate.The results indicated that the Rtin the filtration of pretreated leachate is much lower than that of the raw leachate,while the flux recovery ratio is higher.The Rirreduced Significantly from 47.9%for the raw composting leachate filtration to 19.8%for the filtration of raw leachate.On the other hand,the reversible resistance during the pretreated leachate was higher that of the raw leachate filtration.These results prove that the coagulation pretreatment could remarkably improve the anti-fouling properties of the membrane.Previous studies have demonstrated that organic matter could cause severe membrane fouling;chemical coagulation using PSF was effective for the controlling of membrane fouling.As a result,membrane fouling was rather limited when pre-treatment was carried out prior to the nanofiltration process.
Table 3 Concentration of pollutants in effluent for each treatment process

Fig.6.Summary of FRR,Rir,Rrand Rtof nanofiltration membrane for raw composting leachate and pretreated leachate.
In order to examine the fouling behaviors,the fouled membrane in the filtration of raw leachate and the pretreated leachate was taken out from the membrane cell after the fifth fouling test run without any cleaning and was observed by SEM.As shown in Fig.7(a),an obvious deposition of foulants on the membrane surface was observed and membrane fouling was particularly severe when no pretreatment was applied,as shown in Fig.7(b).While considerable improvement could be observed when chemical coagulation was used as pretreatment,it is consistent for the FRR,Rirand Rrresults.
4.Conclusions
The leachate obtained from a composting reactor was treated successfully by a chemical coagulation and nanofiltration combined process.During the chemical coagulation stage,the removal efficiency reached to 75.3%and 62.8%for turbidity and COD removal,respectively,when the optimum dosage of PFS was 1200 mg·L?1at pH 6.
For the nanofiltration process,about 89.7%of COD,78.2%of TOC,72.5%of TN,83.2%of TP,and 78.6%of NH3-N were retained and the final leachate effluent concentration of COD,NH3-N,TOC and SS was 92 mg·L?1,21 mg·L?1,73 mg·L?1and 23 mg·L?1,respectively.The combination of coagulation– filtration exhibited outstanding treatment performances and this multistage process is useful for composting leachate treatment.
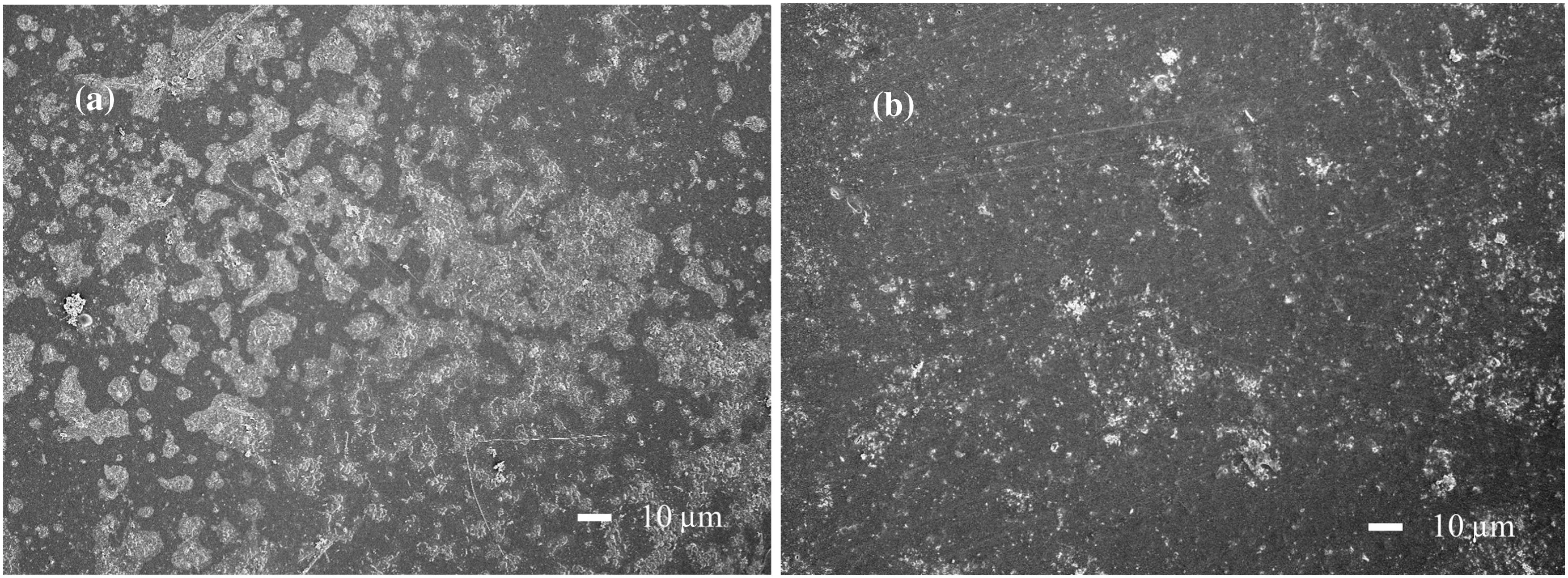
Fig.7.SEM images of fouled PMIA nano filtration membrane surface for the raw composting leachate filtration(a)and chemical coagulation pretreated leachate(b).
[1]A.Baun,A.Ledin,L.A.Reitzel,P.L.Bjerg,T.H.Christensen,Xenobiotic organic compounds in leachates from ten Danish MSW land fills—Chemical analysis and toxicity tests,Water Res.38(2004)3845–3858.
[2]S.Q.Aziz,H.A.Aziz,M.S.Yusoff,M.J.K.Bashir,Land fill leachate treatment using powdered activated carbon augmented sequencing batch reactor(SBR)process:Optimization by response surface methodology,J.Hazard.Mater.189(2011)404–413.
[3]F.Wang,D.W.Smith,M.El-Din,Application of advanced oxidation methods for land fill leachate treatment:A review,J.Environ.Eng.Sci.2(2003)413–427.
[4]L.Bu,K.Wang,Q.L.Zhao,L.L.Wei,J.Zhang,J.C.Yang,Characterization of dissolved organic matter during land fill leachate treatment by sequencing batch reactor,aeration corrosive cell-Fenton,and granular activated carbon in series,J.Hazard.Mater.179(2010)1096–1105.
[5]N.Calace,A.Liberatori,B.M.Petronio,M.Pietroletti,Characteristics of different molecular weight fractions of organic matter in land fill leachate and their role in soil sorption of heavy metals,Environ.Pollut.113(2001)331–339.
[6]M.M.Jessica,F.Markus,I.Hamid,M.F.Patrick,H.Curtis,G.C.Craig,Q.S.Zhen,Intermittent rainstorms cause pulses of nitrogen,phosphorus,and copper in leachate from compost in bioretention systems,Sci.Total Environ.537(2015)294–303.
[7]D.Cassano,A.Zapata,G.Brunetti,G.Del Moro,C.Di Iaconi,I.Oller,S.Malato,G.Mascolo,Comparison of several combined/integrated biological-AOPs setups for the treatment of municipal land fill leachate:Minimization of operating costs and ef fl uent toxicity,Chem.Eng.J.172(2011)250–257.
[8]B.Kayleigh,J.G.Avik,H.Jillian,F.James,Z.Anthony,Membrane bioreactor technology:A novel approach to the treatment of compost leachate,Waste Manag.33(2013)2188–2194.
[9]C.Rafaela,M.Albert,M.Oriol,Nitri fi cation of leachates from manure composting under field conditions and their use in horticulture,Waste Manag.44(2015)72–81.
[10]W.Dastyar,T.Amani,S.Elyasi,Investigation of affecting parameters on treating high-strength compost leachate in a hybrid EGSB and fixed-bed reactor followed by electrocoagulation– flotation process,Process Saf.Environ.95(2015)11–21.
[11]G.Huang,Q.Wu,J.Wong,B.Nagar,Transformation of organic matter during cocomposting of pig manure with sawdust,Bioresour.Technol.97(2006)1834–1842.
[12]T.Carballo,M.V.Gil,X.Gómez,F.González-Andrés,A.Morán,Characterization of different compost extracts using Fourier-transform infrared spectroscopy(FTIR)and thermal analysis,Biodegradation 19(2008)815–830.
[13]J.R.Asztalos,Y.Kim,Enhanced digestion of waste activated sludge using microbial electrolysis cells at ambient temperature,Water Res.87(2015)503–512.
[14]H.Timur,I.Ozturk,Anaerobic sequencing batch reactor treatment of land fill leachate,Water Res.33(1999)3225–3230.
[15]J.Ye,Y.Mu,X.Cheng,D.Sun,Treatment of freshleachate with highstrength organics and calcium from municipal solid waste incineration plant using UASB reactor,Bioresour.Technol.102(2010)5498–5503.
[16]M.Timo,D.N.Long,Land fill leachate treatment using hybrid coagulation–nano filtration processes,Desalination 250(2010)677–681.
[17]S.Cortez,P.Teixeira,R.Oliveira,M.Mota,Mature land fill leachate treatment by denitrification and ozonation,Process Biochem.46(2011)148–153.
[18]H.Zhang,H.J.Choi,C.P.Huang,Treatment of land fill leachate by Fenton's reagent in a continuous stirred tank reactor,J.Hazard.Mater.136(2006)618–623.
[19]S.J.Lim,T.H.Kim,Applicability and trends of anaerobic granular sludge treatment processes,Biomass Bioenergy 60(2014)189–202.
[20]D.Trujillo,X.Font,A.Sánchez,Use of Fenton reaction for the treatment of leachate from composting of different wastes,J.Hazard.Mater.138(2006)201–204.
[21]B.Crouse,A.J.Ghoshdastidar,A.Z.Tong,The presence of acidic and neutral drugs in treated sewage effluents and receiving waters of the Cornwallis and Annapolis River watersheds and Mill Cove WPCC in Nova Scotia,Environ.Res.112(2012)92–99.
[22]S.Kheradmand,A.Karimi-Jashni,M.Sartaj,Treatment of municipal land fill leachate using a combined anaerobic digester and activated sludge system,Waste Manag.30(2010)1025–1031.
[23]H.B.Li,W.Y.Shi,Y.F.Zhang,R.Zhou,H.X.Zhang,Preparation of hydrophilic PVDF/PPTA blend membranes by in situ polycondensation and its application in the treatment of land fill leachate,Appl.Surf.Sci.346(2015)134–146.
[24]S.Eoin,J.Michael,Semmens,E.C.,Performance analysis of a pilot-scale membrane aerated bio film reactor for the treatment of land fill leachate,Chem.Eng.J.273(2015)120–129.
[25]K.S.Shrawan,M.M.Chris,G.Timothy,Townsend ozonation pretreatment for stabilized land fill leachate high-pressure membrane treatment,Desalination 344(2014)163–170.
[26]J.Huang,K.S.Zhang,The high flux poly(m-phenylene isophthalamide)nano filtration membrane for dye purification and desalination,Desalination 282(2011)19–26.
[27]N.E.P.A.Chinese,Water and wastewater monitoring methods,third ed.Chinese Environmental Science Publishing House,Beijing,China,1997.
[28]R.J.Stephenson,S.J.B.Duff,Coagulation and precipitation of a mechanical pulping effluent— I.Removal of carbon,colour and turbidity,Water Res.30(1996)781–792.
[29]J.S.Guo,A.A.Abbas,Y.P.Chen,Z.P.Liu,F.Fang,P.Chen,Treatment of land fill leachate using a combined stripping,Fenton,SBR,and coagulation process,J.Hazard.Mater.178(2010)699–705.
[30]A.A.Tatsi,A.I.Zouboulis,K.A.Matis,P.Samaras,Coagulation– flocculation pretreatment of sanitary land fill leachates,Chemosphere 53(2003)737–747.
[31]C.R.Wu,S.H.Zhang,D.L.Yang,X.G.Jian,Preparation,characterization and application of a novel thermal stable composite nanofiltration membrane,J.Membr.Sci.326(2009)429–434.
[32]A.Y.Zahrim,C.Tizaoui,N.Hilal,Coagulation with polymers for nanofiltration pretreatment of highly concentrated dyes:A review,Desalination 266(2011)1–16.
[33]J.Huang,K.S.Zhang,K.Wang,Z.L.Xie,L.Bradley,H.T.Wang,Fabrication of polyether sulfone-mesoporous silica nanocomposite ultra filtration membranes with antifouling properties,J.Membr.Sci.423-424(2012)362–370.
 Chinese Journal of Chemical Engineering2016年10期
Chinese Journal of Chemical Engineering2016年10期
- Chinese Journal of Chemical Engineering的其它文章
- CFD modeling of a headbox with injecting dilution water in a central step diffusion tube☆
- Interactions between two in-line drops rising in pure glycerin☆
- Hydrodynamics of three-phase fluidization of homogeneous ternary mixture in a conical conduit—Experimental and statistical analysis
- Adsorption of Hg(II)from aqueous solution using thiourea functionalized chelating fiber☆
- Nickel(II)removal from water using silica-based hybrid adsorbents:Fabrication and adsorption kinetics☆
- Reactive dividing wall column for hydrolysis of methyl acetate:Design and control☆
