Hydrodynamic cavitation as an efficient method for the formation of sub-100 nm O/W emulsions with high stability
Zhiliang Zhang*,Guangquan Wang,Yong Nie,Jianbing Ji
Zhejiang Provincal Key Laboratory of Biofuel,College of Chemical Engineering,Zhejiang University of Technology,Hangzhou 310014,China
1.Introduction
Emulsion is a dispersion consisting of at least two immiscible liquids,with one of the liquids being dispersed as small,spherical droplets in the other[1].Nowadays,a growing interest has been attracted on utilization of emulsions in food[1,2],cosmetic[3],pharmaceutical[4],and chemical industries[5].The properties of emulsions depend on their droplet size and size distribution[6–8].Preparation of emulsions with small droplet size and high stability is attractive for both theoretical and practical point of view[6].
Generally,two approaches are employed for the formation of emulsions:high-energy approaches and low-energy approaches[1].The high-energy approaches include high-pressure homogenization[9,10],colloid mills[11],and sonication[5,12,13].Emulsions produced by these techniques had high stability[14,15].However,high-energy approaches are usually associated with high-energy input,making them unfavorable for industrial applications[6,16].Viewing the drawbacks of the high-energy approaches,several novel methods with low-energy consumption have been investigated for the formation of emulsions,such as membrane emulsification[17]and microchannel emulsification[18].The membrane emulsification gains the advantage on controlling droplet size distribution by membrane pore and is suitable for shear-or temperature-sensitive materials,such as starch and proteins[17].The major limiting factor of this practice is the low dispersed phase flux [17,19].The microchannel emulsification,usually implemented by a tube-in-tube microchannel reactor,has a great potential for the high throughput emulsification of emulsions[18].Nevertheless,the formed emulsions are larger than 10 μm and exhibit a poor controlling of the droplet size.
Hydrodynamic cavitation, a newly developed process intensification technique,has recently demonstrated a great potential for formation of emulsions[20–22].Hydrodynamic cavitation is generated when a moving liquid passes through constriction geometries(e.g.orifice and venture)[23].It has a good prospect in industrial application for its easy scale-up and low energy consumption[24–26].However,emulsions prepared by hydrodynamic cavitation are in the order of hundreds of nanometers rather than sub-100 nmsofar.In this work,we applied hydrodynamic cavitation as an efficient method for the formation of sub-100 nm O/W emulsions with high stability.Re fined soybean oil was used as a typical oil phase to prepare O/Wemulsions.The effects of process parameters(i.e.the inlet pressure,the number of cavitation passes and the surfactant concentration)on the droplet size of emulsions and its stability were investigated in detail.
2.Experimental
Re fined soybean oil(TESCO,Hangzhou,China),heptane and castor oil(Aladdin,Shanghai,China)were tested as oil phase.Deionized water was water phase.Sodium dodecyl sulfate(SDS,Aladdin,Shanghai,China)was selected as stabilizer.

Fig.1.The schematic diagram of the hydrodynamic cavitation emulsification process.
The schematic diagram of the process was shown in Fig.1.The hydrodynamic cavitation was generated by a valve with the diameter of 3 mm.By controlling the valve opening,the inlet pressure could be controlled.In a typical experiment,200 ml of oil was mixed with 2 L of water(1.25 wt%SDS)in a tank.The mixture was pumped into the valve by a pump(120 psi).O/W emulsion was formed after the mixture passed through the valve.
The droplet size distribution and zeta potential of the emulsions were measured using a dynamic light scattering instrument(Zetasizer-nano ZS90,Malvern).Samples were diluted appropriately before the droplet size measurements using deionized water.All samples were analyzed in triplicate. The Z-average diameter was reported as the average droplet size [12,13]. The polydispersity index(PDI),which reflected the quality of distribution of the droplet size in an emulsion,was recorded in the measurement.Zeta potential value is an index of the stability of emulsions.When the absolute value was larger than 30 mV,the system was considered to be stable.
3.Results and Discussion
The hydrodynamic cavitation emulsification process was exemplified using re fined soybean oil as oil phase.In this case,the experiment was carried out at the inlet pressure of 100 psi(1 MPa=145 psi).SDS was dissolved in water at the concentration of 1.25 wt%.As shown in Fig.2,milk like emulsion was formed.The average droplet size of the emulsion was 100 nm,which was much smaller than the reported droplet size(>10 μm)[18],demonstrating a successful hydrodynamic cavitation emulsification.

Fig.2.Droplet size distribution of O/W emulsion formed by hydrodynamic cavitation emulsification(inlet pressure:100 psi;SDS concentration:1.25 wt%;number of cavitation passes:1).Insert is the image of emulsion.
The inlet pressure is one of the most important parameters which affect the cavitating condition inside the cavitating device[25].To investigate the effect of inlet pressure,a series of emulsions were prepared by varying the inlet pressure from 20 to 150 psi.As shown in Fig.3,there was an obvious decrease in droplet size when the inlet pressure increased from 20 psi to 120 psi.Emulsion with an average size of 27 nm was formed at120psi.Zeta potential measurement showed negatively charged emulsion(34.0±0.7 mV),which implies a stable system[27,28].However,a further increase of inlet pressure to 150 psi,shows no Significant decrease in the drop size.It is worthy of noting that the polydispersity indexes of the emulsions were lower than 0.1 when the inlet pressure was larger than 120 psi(Table 1),demonstrating good monodispersity of emulsions.

Fig.3.Effect of inlet pressure on droplet size(SDS concentration:1.25 wt%;number of cavitation passes:6).
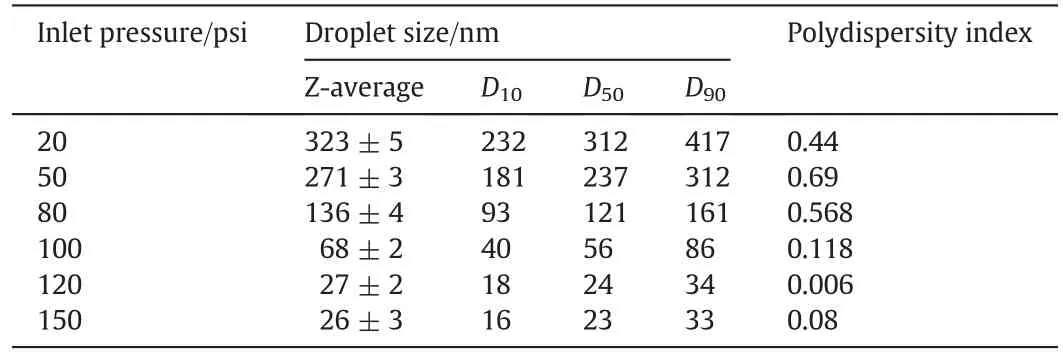
Table 1 Droplet size distributions of emulsions prepared under different inlet pressures(SDS concentration:1.25 wt%;number of cavitation passes:6)
The decreasing of droplet size with the increasing inlet pressure can be attributed to the following reasons.Increasing in the inlet pressure results in an reinforcement of the collapse intensity of cavity,due to which the magnitude of pressure pulse generated was enhanced,favoring the mass transfer between the two immiscible phases(i.e.oil and water)[29].Similar effects have been reported by Braeutigam[24]and Ghayal[30].The emulsion size did not change appreciably after 150 psi,possibly due to the elimination of mass transfer resistance under higher operating pressure[29].
Premix quality is an important factor influencing the quality of an emulsion[9].Passing emulsion through the cavitating device for more than one pass can improve the premix.As shown in Fig.4,the droplet size decreased with the increase of cavitation passes.With one cavitation pass,the average emulsion size was about 140 nm.Increasing the cavitation pass to 6,the emulsion size dramatically decreased to 27nm.This would be expected because the increase of cavitation passes allowed the increase of the energy input for emulsification.

Fig.4.Effect of number of cavitation passes on droplet size(inlet pressure:120 psi;SDS concentration:1.25 wt%).
Surfactants influence the emulsion formation,which help to control the emulsion size by reducing the interfacial tension and reducing agglomeration by affecting interfacial mobility[31].SDSwas used as stabilizer in this study,which acts as a protecting colloid during the emulsion formation, resulting in stabilized emulsion droplets. As shown in Fig. 5,there was a decrease in the droplet size with the increase of SDS concentrations.At low concentrations below 0.75 wt%, formation of relatively large oil drops was obtained. Increasing the concentration to 1.25%,the average droplet size decreased to 27 nm. Such results were similar to the results of the previous publication, and the reason could be attributed to the decrease of the interfacial tension and interfacial mobility[18].

Fig.5.Effect of SDS concentration on droplet size(inlet pressure:120 psi;number of cavitation passes:6).
The primary limitation for developing emulsions practical applications is the relatively low stability[6].The stability of emulsions is highly dependent on the structure but also the method used to prepare them[6,16].The droplet size distributions of the emulsions formed by highenergy approaches can remain unchanged over months[14,15].But the major disadvantage is the high energy cost[16].To assess the stability of emulsions prepared by the hydrodynamic cavitation emulsification method,the droplet size was recorded as a function of storage time at room temperature.
As summarized in Fig.6,the average droplet size of the emulsion has not obviously changed over 8 months,exhibiting a very good physical stability.The stability of the emulsion could essentially satisfy the stability requirements in most practical applications. More importantly, the hydrodynamic cavitation emulsification method can be generalized to fabricate a large variety of O/Wemulsions, such as heptane/water emulsion(68 nm), castor oil/water emulsion (19 nm), as shown in Fig. 7.Consequently, hydrodynamic cavitation emulsification is an efficient method for the formation of high-stability O/W emulsions.

Fig.6.Droplet size as a function of time for the emulsion stored at room temperature.

Fig.7.Droplet size distributions of heptane/water emulsion and castor oil/water emulsion.Inserts are the images of the emulsions.
4.Conclusions
In summary,we have demonstrated the hydrodynamic cavitation as an efficient method for the formation of sub-100 nm O/W emulsions with high stability.The effects of inlet cavitation pressure,number of cavitation passes and surfactant concentration on droplet size of the O/W emulsions were investigated.With the increase of inlet pressure,number of cavitation passes and surfactant concentration,the average droplet sizes of the O/W emulsions were decreased.At the inlet pressure of 120 psi,emulsion with the average size of 27 nm was formed after 6 cavitation passes.The formed O/W emulsion exhibited a very good physical stability during 8 months.The hydrodynamic cavitation emulsification has great potential for the formation of emulsions because it offers the benefit of lower energy consumption and easy to scale up.
[1]D.J.Mcclements,Edible nanoemulsions:Fabrication,properties,and functional performance,Soft Matter 7(6)(2011)2297–2316.
[2]C.Qian,D.J.Mcclements,Formation of nanoemulsions stabilized by model foodgrade emulsifiers using high-pressure homogenization:Factors affecting particle size,Food Hydrocolloids 25(5)(2011)1000–1008.
[3]P.Glampedaki,V.Dutschk,Stability studies of cosmetic emulsions prepared from natural products such as wine,grape seed oil and mastic resin,Colloids Surf.A Physicochem.Eng.Asp.460(0)(2014)306–311.
[4]N.Kiss,G.Brenn,H.Pucher,et al.,Formation of O/W emulsions by static mixers for pharmaceutical applications,Chem.Eng.Sci.66(21)(2011)5084–5094.
[5]S.M.T.Gharibzahedi,S.H.Razavi,S.M.Mousavi,Ultrasound-assisted formation of the canthaxanthin emulsions stabilized by arabic and xanthan gums,Carbohydr.Polym.96(1)(2013)21–30.
[6]L.Yu,C.Li,J.Xu,et al.,Highly stable concentrated nanoemulsions by the phase inversion composition method at elevated temperature,Langmuir 28(41)(2012)14547–14552.
[7]C.Solans,P.Izquierdo,J.Nolla,et al.,Nano-emulsions,Curr.Opin.Colloid Interface 10(3–4)(2005)102–110.
[8]A.Khalil,F.Puel,Y.Chevalier,et al.,Study of droplet size distribution during an emulsification process using in situ video probe coupled with an automatic image analysis,Chem.Eng.J.165(3)(2010)946–957.
[9]J.Floury,A.Desrumaux,J.Lardières,Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsions,Innovative Food Sci.Emerg.Technol.1(2)(2000)127–134.
[10]S.Lee,T.Lefèvre,M.Subirade,et al.,Effects of ultra-high pressure homogenization on the properties and structure of interfacial protein layer in whey proteinstabilized emulsion,Food Chem.113(1)(2009)191–195.
[11]J.M.Perrier-Cornet,P.Marie,P.Gervais,Comparison of emulsification efficiency of protein-stabilized oil-in-water emulsions using jet,high pressure and colloid mill homogenization,J.Food Eng.66(2)(2005)211–217.
[12]K.A.Ramisetty,A.B.Pandit,P.R.Gogate,Ultrasound assisted preparation of emulsion of coconut oil in water:Understanding the effect of operating parameters and comparison of reactor designs,Chem.Eng.Process.Process Intensif.88(0)(2015)70–77.
[13]S.Y.Tang,P.Shridharan,M.Sivakumar,Impact of process parameters in the generation of novel aspirin nanoemulsions—Comparative studies between ultrasound cavitation and micro fluidizer,Ultrason.Sonochem.20(1)(2013)485–497.
[14]T.J.Wooster,M.Golding,P.Sanguansri,Impact of oil type on nanoemulsion formation and Ostwald ripening stability,Langmuir 24(22)(2008)12758–12765.
[15]J.N.Wilking,C.B.Chang,M.M.Fryd,et al.,Shear-induced disruption of dense nanoemulsion gels,Langmuir 27(9)(2011)5204–5210.
[16]M.M.Fryd,T.G.Mason,Advanced nanoemulsions,Annu.Rev.Phys.Chem.63(2012)493–518.
[17]S.M.Joscelyne,G.Tr?g?rdh,Membrane emulsification — A literature review,J.Membr.Sci.169(1)(2000)107–117.
[18]T.Li,Y.Zhou,J.Wang,et al.,High-throughput emulsification in a microporous tubein-tube microchannel device:O/W emulsion formation,Chem.Eng.J.228(0)(2013)155–161.
[19]D.M.Lloyd,I.T.Norton,F.Spyropoulos,Processing effects during rotating membrane emulsification,J.Membr.Sci.466(0)(2014)8–17.
[20]S.Y.Tang,M.Sivakumar,A novel and facile liquid whistle hydrodynamic cavitation reactor to produce submicron multiple emulsions,AICHE J.59(1)(2013)155–167.
[21]S.Parthasarathy,Y.T.Siah,S.Manickam,Generation and optimization of palm oilbased oil-in-water(O/W)submicron-emulsions and encapsulation of curcumin using a liquid whistle hydrodynamic cavitation reactor(LWHCR),Ind.Eng.Chem.Res.52(34)(2013)11829–11837.
[22]K.A.Ramisetty,A.B.Pandit,P.R.Gogate,Novel approach of producing oil in water emulsion using hydrodynamic cavitation reactor,Ind.Eng.Chem.Res.53(42)(2014)16508–16515.
[23]K.S.Suslick,M.M.Mdleleni,J.T.Ries,Chemistry induced by hydrodynamic cavitation,J.Am.Chem.Soc.119(39)(1997)9303–9304.
[24]K.P.Senthil,K.M.Siva,A.B.Pandit,Experimental quantification of chemical effects of hydrodynamic cavitation,Chem.Eng.Sci.55(9)(2000)1633–1639.
[25]V.K.Saharan,A.B.Pandit,P.S.Satish Kumar,et al.,Hydrodynamic cavitation as an advanced oxidation technique for the degradation of acid red 88 dye,Ind.Eng.Chem.Res.51(4)(2012)1981–1989.
[26]J.S.Raut,S.D.Stoyanov,C.Duggal,et al.,Hydrodynamic cavitation:A bottom-up approach to liquid aeration,Soft Matter 8(17)(2012)4562–4566.
[27]D.Li,M.B.Mueller,S.Gilje,et al.,Processable aqueous dispersions of graphene nanosheets,Nat.Nanotechnol.3(2)(2008)101–105.
[28]D.H.Everett,Basic principles of colloid science[M],Royal Society of Chemistry,1988.
[29]D.Ghayal,A.B.Pandit,V.K.Rathod,Optimization of biodiesel production in a hydrodynamic cavitation reactor using used frying oil,Ultrason.Sonochem.20(1)(2013)322–328.
[30]P.Braeutigam,M.Franke,Z.L.Wu,et al.,Role of different parameters in the optimization of hydrodynamic cavitation,Chem.Eng.Technol.33(6)(2010)932–940.
[31]X.Bernat,E.Piacentini,F.Bazzarelli,et al.,Ferrous ion effects on the stability and properties of oil-in-water emulsions formulated by membrane emulsification,Ind.Eng.Chem.Res.49(8)(2010)3818–3829.
 Chinese Journal of Chemical Engineering2016年10期
Chinese Journal of Chemical Engineering2016年10期
- Chinese Journal of Chemical Engineering的其它文章
- CFD modeling of a headbox with injecting dilution water in a central step diffusion tube☆
- Interactions between two in-line drops rising in pure glycerin☆
- Hydrodynamics of three-phase fluidization of homogeneous ternary mixture in a conical conduit—Experimental and statistical analysis
- Adsorption of Hg(II)from aqueous solution using thiourea functionalized chelating fiber☆
- Nickel(II)removal from water using silica-based hybrid adsorbents:Fabrication and adsorption kinetics☆
- Reactive dividing wall column for hydrolysis of methyl acetate:Design and control☆
