Rapid synthesis of CNTs@MIL-101(Cr)using multi-walled carbon nanotubes(MWCNTs)as crystal growth accelerator☆
Qing Wang,Shengqiang Wang*,Hongbing Yu
1College of Environmental Science and Engineering,Nankai University,Tianjin 300071,China
2School of Chemistry and Chemical Engineering,Xinjiang Normal University,Xinjiang 830054,China
1.Introduction
Metal–Organic Frameworks(MOFs)materials,the hybrids of transitionmetal ions and organic multidentate ligands,have the characterization of high surface area,large pore volume,adjustable pore size and diverse cage structure.The intensive coordinate bonds between metal ions and ligands enable MOFs materials having chemical and high temperature stability[1,2].Unlike the pore structures of traditional porous materials,such as sieves,without wall structure,the pores of MOFs materials are derived from the connection of molecular conjugate just like scaffolds.Furthermore,the constructions of MOFs materials with different skeletons make the internal spaces having diverse pore forms.As one kind of new multifunctional materials,the MOFs have extensive applications,including gas storage[3–5],selective adsorption[6],reaction catalysis[7–9],fuel desulfurization[10]and drug delivery[11–15].
MIL-101(Cr) (MIL=Matrial Institut Lavoisier)was first synthesized by Férey with hydrothermal method at 220 °C for 8 h [16], having extra high surface area, large pore volume, and high thermal and chemical stability to water and most organic solvents. It has two types of mesoporous cages(2.9 and 3.4 nm)accessible through microporous windows(1.2 and 1.4 nm).It also has hydrophobic and hydrophilic groups on the surfaces in the pore structure.These outstanding features make it an interesting candidate for adsorption,separation and purification,synthesis of fine chemicals and substrate of catalysts or nanoreactors[17–20].To get MOFs with high degree of crystallization,longer time is always needed from a few hours to several days at definite temperatures.The crystallization of MOFs can be seen as a series of consecutive procedures composed of oligomerization,nucleation,crystalline grain,and crystal growth.Oligomerization and nucleation are the processes before the formation of solid crystal grains precipitating from the hydrothermal reaction system, in which mass transfer from the liquid solution to the solid seeding crystals occurs. The crystal growth rate is generally affected by the formation of crystal nucleus and crystalline grain.Amorphous state or loose precipitation will appear if the hydrothermal reaction undergoes too fast or the reaction time is not sufficient. Thus,nucleation and crystalline grain at given conditions determine the crystallinity and size of MOFs crystals. The crystal size is affected not only by the quantity of crystal nucleuses but also the rate of crystal growth rate. Smaller sized crystals will be obtained in the presence of more crystal nucleuses resulting from the slow crystal growth rate.Otherwise, larger sized crystals will appear which results from the fast crystal growth rate if less crystal nucleuses present in the hydrothermal reaction system. The formation and quantity of crystal nucleusesmay be the main barriers blocking the fast growth of MOFs crystals. According to previous research results, intensified by adding particular nano materials into the hydrothermal reaction systemor heating the reaction solvents with microwaves[21–26],the nucleation formation processes are accelerated and then less time is needed for the consequent crystal growth.Thus smaller crystal particles can be formed in the presence of more crystal nucleuses.Therefore,the obstacles in control of the size and growth rate of MOFs crystals can be broken through in the presence of exogenous inclusions or along with strengthened energy provided byexternal assistance in heterogeneous nucleation.Expanded graphite(EG),as structure-directing template,has been studied for this purpose in the fast synthesis of MIL-101[21,22].The reason for EG in control of the size and growth rate of MOFs crystals was attributed to the oxygen-containing groups on the surface of EG,such as–COOH,?CO,?OH,which may play an important role in the acceleration of crystal nucleation.Thus,the crystals with comparative surface area and pore volume[27,28]can be obtained due to the quick formation of coordinate bonds between the metals and ligands in the presence of exogenous inclusion.Therefore,it is very promising for MIL-101 synthesized in shorter crystallization time,possessing the advantages of MIL-101 and MWCNTs,such as high surface area and large mesoporous volumes,to gain more wide usage in variety industrial applications.
In this purpose,the hybrid material CNTs@MIL-101(Cr)was synthesized using MWCNTs as the crystal growth accelerator in which MWCNTs were meanwhile wrapped in the crystals of MIL-101.The hydrothermal reaction condition of CNTs@MIL-101(Cr)was similar with that of MIL-101.The characteristic differences between the crystals of MIL-101 and CNTs@MIL-101(Cr)were investigated by N2 adsorption–desorption isotherms, X-ray diffraction (XRD) and scanning electron microscope (SEM). The role of MWCNTs played in the fast synthesis of CNTs@MIL-101(Cr) was also discussed in the proposed mechanism.
2.Experimental Sections
2.1.Materials
Fluorhydric acid(HF,48%),1,4-benzenedicarboxylic acid(H2BDC),chromic nitrate nonahydrate(Cr(NO3)3·9H2O,99%)and acetic acid of analysis grade was purchased from J&K Scientific Company Ltd.(Beijing).N,N-dimethyl formamide(DMF,99%)of analysis grade were purchased from Kemiou Chemical Company(Tianjin).Dehydrated alcohol of analysis grade was purchased from Zhenxing Chemical Company(Tianjin).Ultra-pure water was purchased from Nankai University Water Center.MWCNTs were purchased from Nanjing XFNANO Materials Tech.Co.Ltd.
2.2.Synthesis of MIL-101
Two kinds of mineralization agents, fluorhydric acid and acetic acid,were used as mineralizing agent in the synthesis of MIL-101 with hydrothermal method for comparison.Firstly,one solution consisted of H2BDC(1660 mg at 10 mmol),Cr(NO3)3·9H2O(4000 mg at 10 mmol), fluorhydric acid(0.36 ml at 10 mmol),and H2O(48 ml at 2650 mmol)was poured into a 100 ml Te fl on lined stainless steel autoclave for the synthesis of MIL-101.In another reaction solution,acetic acid(7.5 ml at 45.4 mmol)was used as mineralization agent instead of fluorhydric acid and other components were consisted with that of previous one.The autoclaves were sealed and subjected to heat treatmentin an air dry oven at220°Cfor8h.Then,the two samples obtained were collected by centrifugation and then washed the samples with abundant DMF and dehydrated alcohol at 70°C to dissolve the H2BDC in the raw MIL-101 materials and separated them via centrifugation.This process was repeated five times to remove the unreacted H2BDC as far as possible, finally dried the sample vacuum drying oven at 150°C for 12 h.Thus,highly crystallized green powders of MIL-101 with formula Cr3F(H2O)2OE(O2C)-C6H4-(CO2)3·nH2O(where n is~25)based on chemical analysis were produced.
2.3.Rapid synthesis of CNTs@MIL-101(Cr)
In the rapid synthesis of CNTs@MIL-101(Cr),100 mg,300 mg and 500 mg MWCNTs were added into the hydrothermal reaction solution,respectively.Other components were consisted with that of MIL-101 using acetic acid as mineralization agent.After maintaining constant temperature at 220°C for 2 h,the unreacted MWCNTs in the reaction system were removed from the mixture through a conical funnel.Then,the crystals of CNTs@MIL-101(Cr)were purified with centrifugation followed by washing with DMF and dehydrated alcohol for five times at 70°C.The crystals of CNTs@MIL-101(Cr)were obtained after being dried in vacuum condition at 150°C for 12 h.
2.4.Characterization of MIL-101and CNTs@MIL-101(Cr)
The pore properties of MIL-101 and CNTs@MIL-101(Cr)were examined with N2adsorption–desorption isotherms at ?196 °C(ASAP2020,Micromeritics Instrument Corp.,USA).The characterization of MIL-101 and CNTs@MIL-101(Cr)has also been tested by XRD(X-ray Diffractometer,Rigaku D/max 2500v/pc,Rigaku,Japan),SEM(Scanning Electron Microscope,SS-550,Shimadzu,Japan)and TGA(Mettler Toledo,TGA/DSC/1100,Swiss).
3.Results and Discussion
3.1.Characterization of CNTs@MIL-101(Cr),MWCNTs and MIL-101
The N2adsorption and desorption isotherms of CNTs@MIL-101(Cr),MWCNTs and MIL-101 at ?196 °C were shown in Fig.1.The N2adsorption and desorption isotherms of MIL-101 belong to type I,and MWCNTs have the characteristic of type II.However,the N2adsorption and desorption isotherms of CNTs@MIL-101(Cr)meanwhile have the feature of type I and type II indicating the coexistence of micropores and mesopores in CNTs@MIL-101(Cr).
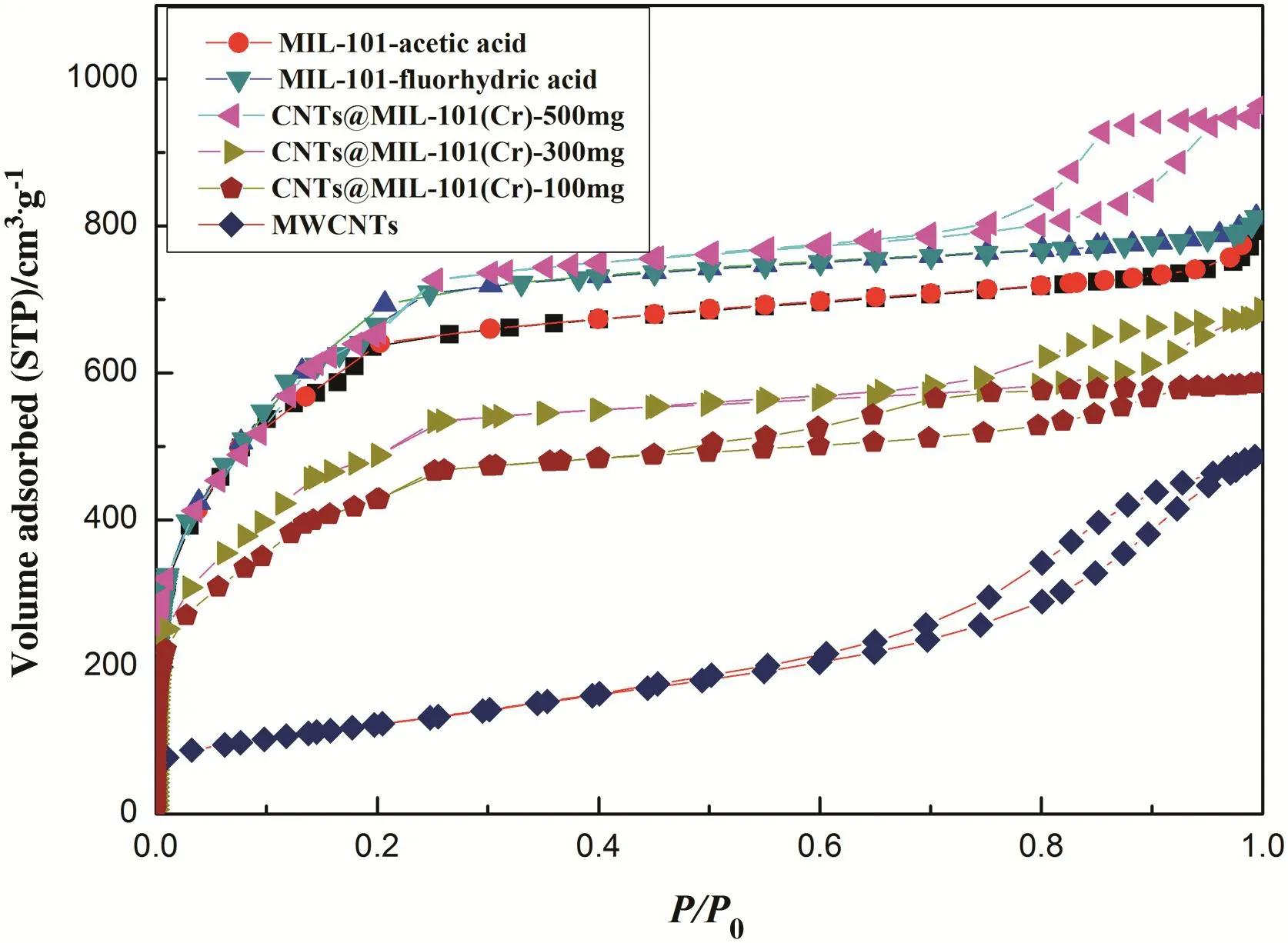
Fig.1.The N2adsorption and desorption isotherms of MIL-101,MWCNTs and CNTs@MIL-101(Cr)at?196 °C.
As shown in Table 1, the BET surface area [29], Langmuir surface area of CNTs@MIL-101(Cr) and MIL-101 were calculated based on the desorption isotherms,respectively.Calculated at the point of p/p0equals to 0.97,the total pore volume of MIL-101( fluorhydric acid and acetic acid)and CNTs@MIL-101(Cr)(500 mg,300 mg,100 mg)was 1.22,1.16,and 1.48,1.05,0.91 cm3·g?1,respectively.By treating the N2adsorption curve data with the BJH method[30],the average pore size of MIL-101( fluorhydric acid and acetic acid)and CNTs@MIL-101(Cr)(500 mg,300 mg,100 mg)were 2.09,2.05,and 2.35,2.32 and 2.31 cm3·g?1,respectively.
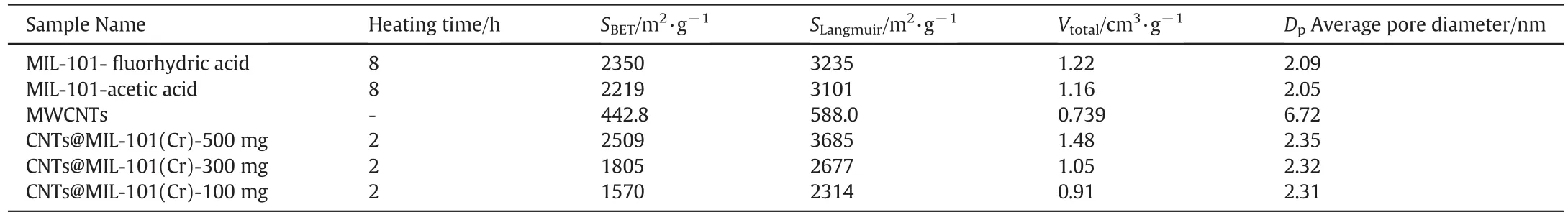
Table 1 The pore characteristics of MIL-101,MWCNTs and CNTs@MIL-101(Cr)
The shape of adsorption isotherms directly correlates with the pore volume and pore size distribution.From the desorption cumulative pore size distribution of MIL-101 and CNTs@MIL-101(Cr)calculated with BJH method in Fig.2,we can see the coexistence of micropores and mesopores in the MOF materials.

Fig.2.BJH desorption cumulative pore volume of MIL-101 and CNTs@MIL-101(Cr).
TGA of CNTs@MIL-101(Cr)-500 mg and MIL-101- fluorhydric acid was tested and the results were shown in Fig. 3.The maximum tolerated temperature of CNTs@MIL-101 and MIL-101- fluorhydric acid was 338 °C and 326 °C which enable the MOFs materials having high temperature stability.

Fig.3.TGA of CNTs@MIL-101(Cr)-500 mg and MIL-101- fluorhydric acid.
The XRD patterns of crystal particles of MIL-101 and CNTs@MIL-101(Cr)were shown in Fig.4.Most XRD patterns from CNTs@MIL-101(Cr)crystals are consistent with that of MIL-101 from its crystal structural data,suggesting that the crystals synthesized in this work are in agreement with that previously reported[16–18].

Fig.4.XRD patterns of MIL-101 and CNTs@MIL-101(Cr).
However,the characteristic XRD pattern of MWCNTs at 26.0°referring to the crystal face d(002)which disappeared in the patterns of CNTs@MIL-101(Cr)crystals.Moreover,the sharpness and intensity of the peak of MWCNTs at 42.4°in the patterns of MWCNTs@MIL-101(Cr)decreased with the increase of adding amount of MWCNTs.With the adding of MWCNTs into the reaction system,the growth speed of CNTs@MIL-101(Cr)seed crystals increases with the rise of adding amount of MWCNTs.The more MWCNTs have been added in the synthetic reaction solution,the better the crystallinity of CNTs@MIL-101(Cr)crystals is,and then the higher the sharpness and intensity of the XRD patterns becomes and the opposite is the other way around.
As presented in Fig.5(a),the MIL-101(Cr)crystals,synthesized in 8h using HF as mineralization agent,have an octahedral shape and the size of which in the range of 400–600 nm.As can be seen from Fig.5(b),the SEM morphology of MIL-101 with acetic acid showed that the MIL-101(Cr)crystals synthesized in eight hours have a smaller size ranging from 300 nm to 500 nm than that of previous crystals at the same condition.As demonstrated in Fig.5(d),after adding MWCNTs(Fig.5(c))in the reaction system,the fast synthesized CNTs@MIL-101(Cr)crystals obtained in 2 h not only preserve an octahedral shape but also have a size in the range of 1.5–2.0 μm which were obviously larger than the crystals of MIL-101 that without using MWCNTs in the reaction system.However,the crystallinity of CNTs@MIL-101(Cr)crystals was on the decrease when the quantity of MWCNTs added in the reaction system reduced.As can be seen from Fig.5(e and f),amorphous crystals will appear if 300 mg or 100 mg MWCNTs was added in the reaction system which means MWCNTs added in the hydrothermal reaction system was not sufficient for the formation of nucleuses.

Fig.5.SEM morphologies of MIL-101,MWCNTs and CNTs@MIL-101(Cr).(a)MIL-101 using HF as mineralization agent,(b)MIL-101 using acetic acid as mineralization agent,(c)SEM morphology of MWCNTs,(d)SEM morphology of CNTs@MIL-101(Cr)using acetic acid as mineralization agent with 500 mg MWCNTs,(e)SEM morphology of CNTs@MIL-101(Cr)using acetic acid as mineralization agent with 300 mg MWCNTs,(f)SEM morphology of CNTs@MIL-101(Cr)using acetic acid as mineralization agent with 100 mg MWCNTs.The scale bar is the interval between the whole white dots.
The reason for the crystal size changes between CNTs@MIL-101(Cr)and MIL-101 was attribute to the highly delocalized π bonds at the out surface of MWCNTs which render H2BDC being easily adsorbed on to MWCNTs and reduce the surface tension of MWCNTs.Thus,nucleation centers were formed on the MWCNTs and meanwhile MWCNTs were embedding in the crystals of MIL-101.After nucleation,CNTs@MIL-101(Cr)crystal grains appear in the form of hybrid material with larger particle size. The results indicate that fast synthesis of MOFs crystals will become possible in shorter time with proper amount of MWCNTs by using hydrothermal method.
3.2.Mechanism
In previous literature, the mechanism for the fast synthesis of MIL-101, using expanded graphite as structure-directing agent,was attributed to the surface oxygen-containing groups on expanded graphite, such as –COOH, ?CO,?OH, which increase the quantity of nucleuses in the reaction system before crystal growth process [21,22]. Thus, the crystals presented smaller dimensions than that without the adding of structure-directing agent. However, in this study, larger crystals were obtained if adding MWCNTs in the reaction solution than that without.Therefore, it can be deduced that the quantity of nucleuses decreased after adding MWCNTs in the reaction system. Therefore the crystal growth rate increased due to fewer nucleuses.
MWCNTs,the coaxial cylindrical tubes composed of multi-layer graphite sheets,can be seen as the external particles in heterogeneous nucleation.On one hand,the highly delocalized π bond at the outside of the graphite sheets is the chemical combination basis between MWCNTs and organic conjugated molecules with non-covalent bond.1,4-Benzenedicarboxylate,having aromatic ring,can be adsorbed on the outside surface of MWCNTs with non-covalent bond and which results in the decrease of surface tension of carbon nanotubes and the increase of their dispersity in the reaction solution.On the other hand,the occurrence of nucleation was not indispensably the formation of sphere nucleuses,but just that of spherical segment.Thus,less activation energy may be needed during the nucleation process in the fast synthesis of CNTs@MIL-101(Cr).After the nucleuses presented leeching onto the out surfaces of MWCNTs.It is an easy-to-build way for the increase of crystal growth rate.Thus,crystals with larger dimensions came into being compared with that synthesized with the traditional method.
4.Conclusions
In this study,we have synthesized metal–organic frameworks CNTs@MIL-101 within 2 h using MWCNTs as the crystal growth accelerator.The crystallization of CNTs@MIL-101(Cr)was affected by the quantity of MWCNTs added in the reaction system.The crystals of CNTs@MIL-101(Cr)using 500 mg MWCNTs has the same octahedral shape in the SEM morphology as that of MIL-101.Amorphous state crystals will be obtained if the quantity of MWCNTs added in the reaction system below 500 mg which means the role MWCNTs played in the reaction being not contributive to the nucleation,but the crystal growth rate.The crystal nucleuses may be hard to form if the quantity of nucleation centers is not sufficient before the formation of solid crystal grains precipitating from the hydrothermal reaction system.Moreover,the large crystal size and mesoporous volume for the fast synthesized CNTs@MIL-101(Cr)can be attributed to the fast crystal growth rate based on MWCNTs embedding in the hybrid material.Then,the crystal growth rate is accelerated and it results in rapid synthesized CNTs@MIL-101(Cr)crystals having larger dimensions in comparison with that of the traditional ones.The rapid synthesis of porous hybrid material CNTs@MIL-101(Cr)via hydrothermal method using embedding MWCNTs as the crystal growth accelerator makes MIL-101 a good candidate for industrial-scale application.

[1]A.K.Cheetham,C.N.R.Rao,R.K.Feller,Structural diversity and chemical trends in hybrid inorganic–organic framework materials,Chem.Commun.46(2006)4780–4795.
[2]O.M.Yaghi,M.O'Keeffe,N.W.Ockwig,H.K.Chae,M.Eddaoudi,J.Kim,Reticular synthesis and the design of new materials,Nature 423(2003)705–714.
[3]M.Latroche,S.Surblé,C.Serre,C.Mellot-Draznieks,P.L.Llewellyn,J.H.Lee,J.S.Chang,S.H.Jhung,G.Férey,Hydrogen storage in the giant-pore metal–organic frameworks MIL-100 and MIL-101,Angew.Chem.Int.Ed.45(2006)8227–8231.
[4]J.Yang,Q.Zhao,J.Li,J.Dong,Synthesis of metal–organic framework MIL-101 in TMAOH-Cr(NO3)3-H2BDC-H2O and its hydrogen-storage behavior,Microporous Mesoporous Mater.130(2010)174–179.
[5]R.E.Morris,P.S.Wheatley,Gas storage in nanoporous materials,Angew.Chem.Int.Ed.47(2008)4966–4981.
[6]J.R.Li,R.J.Kuppler,H.C.Zhou,Selective gas adsorption and separation in metal–organic frameworks,Chem.Soc.Rev.38(2009)1477–1504.
[7]J.Y.Lee,O.K.Farha,J.Roberts,K.A.Scheidt,S.T.Nguyen,J.T.Hupp,Metal–organic framework materials as catalysts,Chem.Soc.Rev.38(2009)1450–1459.
[8]D.Farrusseng,S.Aguado,C.Pinel,Metal–organic frameworks:opportunities for catalysis,Angew.Chem.Int.Ed.48(2009)7502–7513.
[9]H.H.Zhao,H.L.Song,L.J.Chou,Nickel nanoparticles supported on MOF-5:synthesis and catalytic hydrogenation properties,Inorg.Chem.Commun.15(2012)261–265.
[10]K.A.Cychosz,A.G.Wong-Foy,A.J.Matzger,Liquid phase adsorption by microporous coordination polymers:removal of organosulfur compounds,J.Am.Chem.Soc.130(2008)6938–6939.
[11]P.Horcajada,C.Serre,M.Vallet-Regi,M.Sebban,F.Taulelle,G.Férey,Metal–organic frameworks as efficient materials for drug delivery,Angew.Chem.Int.Ed.45(2006)5974–5978.
[12]P.Horcajada,C.Serre,G.Maurin,N.A.Ramsahye,F.Balas,M.Vallet-Regí,M.Sebban,F.Taulelle,G.Férey,Flexible porous metal–organic frameworks for a controlled drug delivery,J.Am.Chem.Soc.130(2008)6774–6780.
[13]K.M.L.Taylor-Pashow,J.Della Rocca,Z.G.Xie,S.Tran,W.B.Lin,Post synthetic modifications of iron-carboxylate nanoscale metal–organic frameworks for imaging and drug delivery,J.Am.Chem.Soc.131(2009)14261–14263.
[14]J.An,S.J.Geib,N.L.Rosi,Cation-triggered drug release from a porous zinc–adeninate metal–organic framework,J.Am.Chem.Soc.131(2009)8376–8377.
[15]P.Horcajada,T.Chalati,C.Serre,B.Gillet,C.Sebrie,T.Baati,J.F.Eubank,D.Heurtaux,P.Clayette,C.Kreuz,J.-S.Chang,Y.K.Hwang,V.Marsaud,P.-N.Bories,L.Cynober,S.Gil,G.Férey,P.Couvreur,R.Gref,Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging,Nat.Mater.9(2010)172–178.
[16]G.Férey,C.Mellot-Draznieks,C.Serre,F.Millange,J.Dutour,S.Surble,I.Mirgiolaki,A chromium terephthalate-based solid with unusually large pore volumes and surface area,Science 309(2005)2040–2042.
[17]S.H.Jhung,J.Lee,J.W.Yoon,C.Serre,G.Férey,J.S.Chang,Microwave synthesis of chromium terephthalate MIL-101 and its benzene sorption ability,Adv.Mater.19(2007)121–124.
[18]Z.X.Zhao,X.M.Li,S.S.Huang,Q.B.Xia,Z.Li,Adsorption and diffusion of benzene on chromium-based metal organic framework MIL-101 synthesized by microwave irradiation,Ind.Eng.Chem.Res.50(2011)2254–2261.
[19]D.Y.Hong,Y.K.Hwang,C.Serre,G.Férey,J.S.Chang,Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites:surface functionalization,encapsulation,sorption and catalysis,Adv.Funct.Mater.19(2009)1537–1552.
[20]G.Férey,Hybrid porous solids:past,present,future,Chem.Soc.Rev.37(2008)191–241.
[21]Z. Ni, R.I. Masel, Rapid production of metal–organic frameworks via microwave-assisted Solvothermal synthesis, J. Am. Chem. Soc. 128 (38) (2006) 12394–12395.
[22]S.H.Jhung,J.H.Lee,J.S.Chang,Microwave synthesis of a nanoporous hybrid material,chromium trimesate,Bull.Kor.Chem.Soc.26(2005)880–881.
[23]S.H.Jhung,J.H.Lee,P.M.Forster,G.Férey,A.K.Cheetham,J.S.Chang,Microwave synthesis of hybrid inorganic–organic porous materials:phase selective and rapid crystallization,Chem.Eur.J.12(2006)7899–7905.
[24]Y.Hu,C.Liu,Y.Zhang,N.Ren,Y.Tang,Microwave-assisted hydrothermal synthesis of nanozeolites with controllable size,Microporous Mesoporous Mater.119(2009)306–314.
[25]S.H.Jhung,T.Jin,Y.K.Hwang,J.-S.Chang,Microwave effect in the fast synthesis of microporous materials:which stage between nucleation and crystal growth is accelerated by microwave irradiation?Chem.Eur.J.13(2007)4410–4417.
[26]N.A.Khan,I.J.Kang,H.Y.Seok,S.H.Jhung,Facile synthesis of nano-sized metal organic frameworks,chromium–benzenedicarboxylate,MIL-101,Chem.Eng.J.166(2011)1152–1157.
[27]L.T.Yang,L.G.Qiu,S.M.Hu,X.Jiang,A.J.Xie,Y.H.Shen,Rapid hydrothermal synthesis of MIL-101(Cr)metal–organic framework nanocrystals using expanded graphite as a structure-directing template,Inorg.Chem.Commun.35(2013)265–267.
[28]M.Jahan,Q.L.Bao,J.X.Yang,K.P.Loh,Structure-directing role of graphene in the synthesis of metal–organic framework nanowire,J.Am.Chem.Soc.132(2010)14487–14495.
[29]S.Brunauer,P.H.Emmett,E.Teller,Adsorption of gases in multimolecular layers,J.Am.Chem.Soc.60(1938)309–319.
[30]E.P.Barrett,L.G.Joyner,P.P.Halenda,The determination of pore volume and area distributions in porous substances.I.Computations from nitrogen isotherms,J.Am.Chem.Soc.73(1951)373–380.
 Chinese Journal of Chemical Engineering2016年10期
Chinese Journal of Chemical Engineering2016年10期
- Chinese Journal of Chemical Engineering的其它文章
- CFD modeling of a headbox with injecting dilution water in a central step diffusion tube☆
- Interactions between two in-line drops rising in pure glycerin☆
- Hydrodynamics of three-phase fluidization of homogeneous ternary mixture in a conical conduit—Experimental and statistical analysis
- Adsorption of Hg(II)from aqueous solution using thiourea functionalized chelating fiber☆
- Nickel(II)removal from water using silica-based hybrid adsorbents:Fabrication and adsorption kinetics☆
- Reactive dividing wall column for hydrolysis of methyl acetate:Design and control☆
