Br?nsted-acidic ionic liquids as catalysts for synthesizing trioxane☆
Yamei Zhao,Yufeng Hu*,Jianguang Qi,Weiting Ma
State Key Laboratory of Heavy Oil Processing and High Pressure Fluid Phase Behavior&Property Research Laboratory,China University of Petroleum,Beijing 102249,China
1.Introduction
Trioxane is the ring trimer of formaldehyde[1–6].Trioxane is an important chemical material that has a wide variety of applications,including the preparation of poly(oxymethylene)(POM)polymers,anhydrous formaldehyde and pesticide,molding material,bonding material,disinfectant agent,antibacterial agent,and so on[1,2].Trioxane can be applied to all reactions involving formaldehyde, and is particularly useful in reactions that use anhydrous formaldehyde as a reactant.
POMs have excellent mechanical properties and high chemical resistance and, therefore, have been used in a wide variety of industries such as car manufacture,machinery,electricity,electronics,instrument,agriculture,manufacture of building materials,light industry,etc.[7,8].The synthesis of POM polymers often needs ultra-clean trioxane as an educt[4].This method accounts for 80%POM products all over the world.The POM market is continuously growing and,therefore,producers are expanding their production facilities[9].For new plants,it is highly desirable to overcome the intrinsic disadvantages of the existing POM process[1,2,9,10].The most problem of the industrial process for the production of POMs is the energy consumption during the production of its monomer trioxane[6].Therefore,it is critically important to increase the concentration and the selectivity of trioxane in a one-pass vaporization[6],as trioxane is removed as distillate from the reaction solution in the distillation tower and the heat of vaporization of the mixture(water-formaldehyde)is much greater than that of trioxane[6].
Trioxane is synthesized from concentrated aqueous formaldehyde solutions in the presence of an acidic catalyst such as sulfuric acid,cation exchange resin having a sulfonic acid group,zeolite or the like[1,2,11].These existing catalysts have various problems associated with corrosion of production equipment,precipitation of para formaldehyde at high formaldehyde concentration,and low trioxane selectivity and large amount of by-products[1,2,11].Particularly,sulfuric acid has been extensively used as a catalyst in production of trioxane since 1960s[6].However,there still exist serious defects,including high corrosivity,large amount of by-products,etc.[1,2,11].The mostharmful by-product is formic acid,which will inevitably form as a consequence of using the acidic catalyst in the trioxane-forming reaction[11].A high formic acid concentration will cause equipment corrosion by formic acid[1,2,11].In order to conduct the trioxane-forming reaction at a high formaldehyde concentration and to decrease the concentration of formic acid in the reaction system,it is necessary to increase methanol concentration in reaction system and also necessary that the concentrations of methanol and formic acid present in the reaction system during reaction are controlled each at 0.5–5.0 wt%[11].However,such a low formic acid concentration requires us to use low reaction temperature and small amount of catalyst.Under these conditions,an efficient synthesis of trioxane is difficult[11].
Ionic liquids(ILs)have been used to replace traditional liquid acids,such as sulfuric acid and hydrochloric acid,in chemical processes[12–16].Recently,ILs have been used as catalysts in preparation of trioxane[2,17–20].The IL catalysts have the following advantages[2,17–20].First,their corrosivity is so low that no special requirement for equipments exists and,second,the trioxane-forming reaction can be conducted at the formaldehyde concentration as high as 80 wt%without precipitation of paraformaldehyde[2,17–20].However,the price of the IL catalyst that has been successfully applied in pilot plant trial is as high as128000 USD per ton[21].Fortunately,ILs have been referred as “designer solvents”[12–15].And the route of the IL catalyzed organic reaction can be tuned by optimization of the cation or the anion of the IL[13].Nonetheless,to utilize the properties of ILs to modulate reactivity and selectivity of the trioxane-forming reaction and,particularly,to find a more economic IL catalyst for trioxane production,it requires us to uncover the mechanisms as how ILs catalyze the trioxane-forming reaction[13].Particularly,the concentration and the selectivity of trioxane in a one-pass vaporization depend strongly on the properties of the corresponding catalyst such as the activity,the selectivity,and the performance as an extraction distillation agent[22].However,the effect of the IL catalyst was evaluated using the composition of the resultant distillate[2,18–20],which emerge as a manifestation of the overall performance of the IL catalyst.Therefore,here the batch reaction experiments and the continuous production experiments are made simultaneously for the first time to uncover the effects of the ionic structures on the activity,the selectivity,and the performance as an extraction distillation agent in the IL catalyzed synthesis of trioxane.
2.Experimental
2.1.Materials and reagents
The analytical grade compounds including 1-octyl-2-pyrrolidinone(NOP),1-cyclohexyl-2-pyrrolidinone(NCyP),p-toluenesulfonic acid hydrate(p-TSA·H2O),benzenesulfonic acid(BSA),trifluoromethanesulfonic acid(TfOH),2,4-dinitrobenzenesulfonic acid hydrate(DNBSA·H2O),methanesulfonic acid(MSA),and 1,3-propanesultone were purchased from Shanghai Jingchun Chemical Co.,Ltd.,Shanghai,China.Sulfuric acid(98 wt%)and aqueous formaldehyde solutions(37–40 wt%)were purchased from Sinopharm Chemical Reagent Co.,Ltd.,Beijing,China.The 37–40 wt%aqueous solutions of formaldehyde were concentrated tohigherconcentrations(e.g.,50,55and60wt%)byvacuumdistillation at 348.15 K.The concentrations of formaldehyde were determined by sodium sulfite method[23].
2.2.Synthesis of Br?nsted-acidic ILs
The procedures for preparing the target ILs are similar to those used in our previous studies[24]and are described briefly as follows.Equi-molar amounts of NCyP and the corresponding acids were mixed slowly using acetone as solvent and stirred for 4–6 h at room temperature.The resulting viscous liquid was homogeneous and light yellow.The mixed solution was dried under vacuum at333.15 K by rotary evaporator so as to remove acetone.Then the remaining solution was cooled to room temperature and there appeared a lot of solid ILs.The products were washed by toluene more than three times to remove residual material.The products were filtered from solution and dried under vacuum for more than 72 h at 343.15 K.The procedure for synthesis of[NOP]+-based ILs was similar to that for preparing[NCyP]+-based ILs.These ILs were dried by 3 ? molecular sieves for more than 2 days before their use.The water content determined by the Karl–Fisher titration was<180 g·g?1.
The yields of the novel Br?nsted-acidic ILs are ~85 wt%.Fresh[NCyP][BSA],[NCyP][p-TSA],and[NCyP][TfO]are stiff solid at room temperature.[NOP][MSA]and[NOP][HSO4]are viscous yellow transparent liquids.[NOP][BSA]is a viscous yellow liquid and[NOP][DNBSA]is a viscous brown liquid at room temperature.These two ILs slowly solidify at room temperature.
Thermal stabilities of the produced ILs were measured by using a TA Q500 TGA analyzer.The TGA measurements were performed in the temperature range of 313.15–673.15 K through using a heating rate of 20 K·min?1and nitrogen as a purge gas.
The decomposition temperatures(Td)of the examined ILs were measured with a TAQ500 TGA analyzer.TA Universal Analysis software was applied to determine the onset temperature(Tonset)which was often regarded as Td[25,26].The results are shown in Table 1(the Tdof[NCyP][BSA]and[NCyP][p-TSA]has been reported[24]).[NCyP][TfO]has the highest Td(555.15 K).[NOP][MSA]earns the lowest Td(452.15 K)and[NOP][HSO4]has the second lowest Tdwith a value of 485.15 K.The values of Tdof the rest ILs range from 513.15 to 525.15 K.
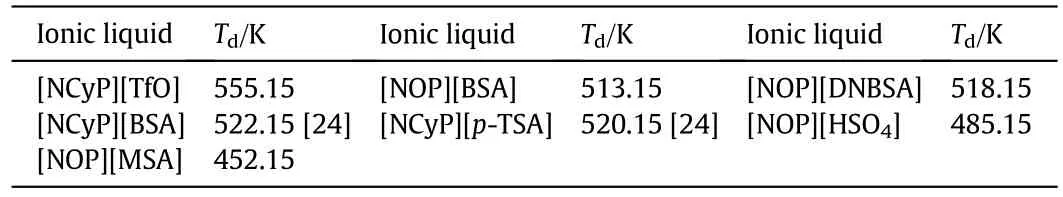
Table 1 Decomposition temperatures(Td)of the novel Br?nsted-acidic ILs
The chemical structures of the produced ILs were shown in Fig.1.A JEOL ECA–600 NMR spectrometer was used to collect1H spectra and operated at 600 MHz.D2O was applied as the solvent and all NMR measurements were performed at room temperature.The spectral data agree well with the structures of the ILs.Spectral data for some Br?nsted-acidic ILs have the following spectral properties(the spectral data of[NCyP][BSA]and[NCyP][p-TSA]have been reported[24]).[NCyP][TfO](D2O):δ=3.7078(m,1H),3.4623(t,2H),2.4148(t,2H),1.9788(m,2H),1.7591(m,2H),1.5968(m,2H),1.4052–1.4315(m,2H),1.2801–1.3019(m,2H),1.0736–1.0954(m,2H).[NOP][DNBSA](D2O):δ=8.6337(s,1H),8.5161(s,1H),8.1989(s,1H),3.4508(t,2H),3.3302(t,2H),2.4458(t,2H),2.0258(t,2H),1.4683(t,2H),1.2445(m,10H),0.8659(t,3H).[NOP][BSA](D2O):δ =7.6619(d,2H),7.3826–7.4262(m,3H),3.2678(t,2H),3.0412(t,2H),2.2507(t,2H),1.8136(t,2H),1.3100(t,2H),1.1195(m,10H),0.6938(t,3H).
2.3.Measurement of the Hammett acidity function H0of aqueous solutions of typical ILs
The Br?nsted acidity was evaluated from the determination of the Hammett acidity functions H0,using UV–visible spectroscopy[27–29].A series of different concentration of ILs solutions were prepared,using p-nitroaniline as an indicator[29].The protonation extent of uncharged indicator bases(named I)in a solution was evaluated in terms of the measurable ratio[I]/[IH+].The Hammett acidity function H0was then calculated using[27–29]:

where pKais the electrolytic constant of the indicator p-nitroaniline(pKa=0.99)and[I]and[HI+]are the percentages of unprotonated and protonated p-nitroaniline,respectively.The results are shown in Table 2.It can be seen that the H0values of aqueous solutions of HCl and of HCl and LiCl measured in this study agree well with those reported in[27].
2.4.Preparation of trioxane
2.4.1.Batch reaction experiments

Fig.1.The structures of novel Br?nsted-acidic ILs:(1)[NCyP][p-TSA],(2)[NOP][BSA],(3)[NCyP][BSA],(4)[NCyP][TfO],(5)[NOP][DNBSA],(6)[NOP][MSA],(7)[NOP][TfO].
The procedure for the batch reaction experiments is similar to that used in our previous measurements[30].A known amount of aqueous formaldehyde solution was added to a 250 ml spherical reactor equipped with a sampling device.A Pt/100 temperature sensor monitored by a CSOOB digital thermometer was inserted into the reactor to control the temperature with an uncertainty of±0.1 K.The reactor is pressurized with nitrogen gas via a conduit system to 1.0 MPa to prevent the solution from boiling.The reactor was then heated in an electric heating jacket with continuous stir.When the temperature reached the pre-settemperature points,an appropriate amount of IL catalyst was mixed with aqueous formaldehyde solution in the reactor and the reaction time was recorded. The cyclotrimerization reaction was typically allowed to proceed for 3–5 h. During the reaction, a known amount of reaction solution was drawn and injected into a chromatography jar at certain intervals(e.g.,0.5,1,1.5,2,2.5,3,and 5 h)to monitor the variations of components in the reaction solutions.At the same time,another known amount of reaction solution was injected into a conical fl ask to determine the acid value of the reaction solutions by acid–base titration.
2.4.2.Continuous production experiments
The procedure for the continuous production experiments is also similar to that used in our previous measurements[30].For the continuous production of trioxane,a 250 ml glass reactor provided with a formaldehyde solution feeder and a packed tower was used. The packedtower was filled by stainless steel raschig rings.A reflux condenser,which was added to the top cover of the packed tower,was heated with warm water at approximately 323.15 K so that the vapor phase condensed on the inner face of reflux condenser without the precipitation of para formaldehyde.A Pt/100 temperature sensor monitored by a CSOOB digital thermometer was inserted into the reactor to control the temperature of reaction with an uncertainty of±0.1 K.The reaction mixture(100 ml)consisting of 50 wt%formaldehyde solution and the acid catalyst was heated in an electric heating jacket.The reaction time was recorded when the temperature reached~375.15 K.After 1 h,a 50 wt%formaldehyde solution(100 ml)was continuously fed to the reactor from the feeder,and the fed rate of formaldehyde solution was adjusted to obtain a constant level of the reaction mixture.A reflux ratio of 2:1 was maintained.When the feed of formaldehyde solution was finished,the distillate was analyzed by gas chromatography with a thermal conductivity detector(TCD).

Table 2 The Br?nsted acid strengths of various ionic liquids①
2.4.3.Vapor–liquid equilibrium experiments
In the present VLE measurements, a CP-I dual circulation vapor–liquid equilibrium still was used,which has been described before[31–33].In operation of the CP-I,about 80 ml of solution containing 50 wt%formaldehyde and 2 wt%acid catalyst was added to the boiling chamber and heated by a heating coil which was controlled by a variac.The cyclotrimerization reactions and VLE were typically allowed to proceed for 5 h.During the reaction,the samples of the equilibrium vapor and liquid phases were taken out from vapor and liquid sampling ports and analyzed at a certain interval to monitor the change with reaction time in the composition of the reaction solution.The concentration of trioxane was analyzed by gas chromatography,and the acid value of the reaction solution was determined by acid–base titration.
3.Results and Discussion
3.1.Batch reaction
Trioxane was firstly produced from a 60 wt%aqueous formaldehyde solution in the presence of 1–3 wt%[NCyP][TfO].The reaction temperature was fixed at~373.15 K.During each experiment the concentration of the produced trioxane and the acidity of the reaction solution as a function of reaction time(tr)were monitored and the results are shown in Fig.2.Both the formation rate of trioxane and the equilibrium yield of trioxane increase with increasing the concentration of[NCyP][TfO].However,the formation rate of the by-product(formic acid)as indicated by the rapidity of increase in the acidity of the reaction solution remains approximately the same.

Fig.2.The yields of trioxane(a)and the acid strength of the reaction solution(b)as a function of reaction time from a 60 wt%aqueous solution of formaldehyde in the presence of different concentrations of[NCyP][TfO]at 373.15 K:□ 1 wt%,○ 2 wt%,and△3 wt%(lines are just a guide to eyes).
The concentrations of methylene glycol trimers(MG3)in dilute aqueous solutions of formaldehyde are always low[6,30].Therefore,the equilibrium yield of trioxane is small.Indeed,Fig.3 shows that both the conversion rate and the equilibrium yield of trioxane increase considerably from 50 to 60 wt%aqueous formaldehyde solutions.However,the increase in the rapidity of conversion and the equilibrium yield of trioxane is smaller as the concentration of formaldehyde is further enhanced from 55 to 60 wt%.Therefore,in the following studies a 60 wt%formaldehyde solution was preferably used.
Trioxane was also produced from a 60 wt%aqueous formaldehyde solution in the presence of 2 wt%[NCyP][TfO]at(369,371,373,and 375)K,respectively.The results are shown in Fig.4.Both the formation rate of trioxane and the equilibrium yield of trioxane increase with increasing the temperature.However,the rate of formation of formic acid also increases as the temperature is increased.The increase in the concentration of trioxane with reaction time is greater but the concentration of formic acid is going up even faster as the temperature exceeds 373 K.Therefore,the following experiments were carried out at 373 K.
The synthesis of trioxane has been made from a 60 wt%aqueous formaldehyde solution in the presence of 2 wt%IL catalysts or sulfuric acid for the purpose of comparisons.The results are shown in Fig.5.Both the formation rate of trioxane and the equilibrium yield of trioxane are greatest when sulfuric acid was used.However,the acidity of the reaction solution is also Significantly increased(by nearly at least 2.5 times)as sulfuric acid was used in place of an IL catalyst.More specifically,it can be seen from Fig.5b that the acidity of the reaction mixture containing sulfuric acid may be as high as 20000 g·g?1.By comparison,the acidity of the reaction mixture involving an IL is always<8000 g·g?1over the experimental reaction time range.

Fig.3.The yields of trioxane(a)and the acid strength of the reaction solution(b)as a function of time using different concentrations of aqueous formaldehyde solutions at 373.15 K:□ 50 wt%,○ 55 wt%,and☆ 60 wt%(using 2 wt%[NCyP][TfO];lines are a guide to eyes).
The conversion rate and the equilibrium yield of trioxane using the[NCyP]+-based IL catalysts decrease in the order[NCyP][TfO]>[NCyP][BSA]?[NCyP][p-TSA],which is inversely related to the H0value of the aqueous solution of these ILs shown in Table 2.Note that the rates of a number of reactions catalyzed by strong acids at high concentrations have been shown to correlate closely with the H0of the acid solutions[27].

Fig.5.The yields of trioxane(a)and the acid strength of the reaction solution(b)as a function of time using different Br?nsted-acidic IL catalysts: □ [NCyP][TfO],○ [NCyP][BSA],☆ [NCyP][p-TSA],● [NOP][BSA],× [NOP][DNBSA],▲ [NOP][MSA],■[NOP][TfO],and+H2SO4(373 K;using 2 wt%IL;lines are a guide to eyes).
In the case of using the[NOP]+-based ILs,the conversion rate and the equilibrium yield of trioxane follow the order[NOP][DNBSA]>[NOP][BSA].It can be seen from Table 3 that the formation rate of formic acid is approximately the same. The conversion rate and the equilibrium yield of trioxane follow the order[NOP][TfO]>[NOP][MSA].This also correlates inversely with the H0of the aqueous solution of these ILs shown in Table 2.

Table 3 The yields of trioxane and formic acid in the reaction solutions of the batch reaction experiments as a function of reaction time(tr)
According to Fig.5 and Table 3,the examined ILs can be classified into three groups:A([NCyP][p-TSA],[NCyP][BSA],[NOP][BSA]),B([NOP][MSA],[NOP][DNBSA]),and C([NOP][TfO],[NCyP][TfO]).The group A corresponds to, first,the smallest(conversion rate and equilibrium yield)of trioxane,second,the smallest acid strength of the reaction solution(i.e.,the concentration of formic acid)up to tr=2 h,and third,the greatest increase with trin the concentration of formic acid at tr>2 h.The group B generally yields(1)the intermediate(conversion rate and equilibrium yield)of trioxane,(2)the intermediate concentration of formic acid up to tr=2 h,and(3)the intermediate increase with trin the concentration of formic acid at tr>2 h.The group C is associated with the functionalized ILs. Functionalization of the anion(from[MSA]?to[TfO]?)gives rise to the greatest(conversion rate and equilibrium yield)of trioxane,the greatest concentration of formic acid over the entire trrange,and the intermediate increase with trin the concentration of formic acid at tr>2 h.
3.2.Continuous production
The continuous production experiments were made using[NOP][MSA],[NOP][DNBSA],and[NCyP][TfO]as the catalyst,respectively.The concentrations of formaldehyde and the catalyst were 60 wt%and 2 wt%,respectively.The results are shown in Table 4 and Fig.6.The best feature of using the[NOP]+-based IL,instead of sulfuric acid, as a catalyst for trioxane synthesis is the comparable yield and much higher selectivity.For example,the yield of trioxane at tr=5 h is 39.72 wt%by[NOP][MSA],48.89 wt%by[NCyP][TfO],and 43.01 wt%by H2SO4.However,the yield of formic acid at tr=5 h is 1654 g·g?1by[NOP][MSA],3180 g·g?1by[NCyP][TfO],and 8576 g·g?1by H2SO4.

Table 4 The yields of trioxane and formic acid in the reaction solutions of the continuous production experiments as a function of reaction time(tr)

Fig.6.The yields of trioxane(a)and acid strength of the reaction solution of the continuous production experiments(b)as a function of time using different Br?nsted-acidic IL catalysts:□ [NCyP][TfO],○[NOP][DNBSA],▲[NOP][MSA]and☆H2SO4(using 2 wt%IL;lines are a guide to eyes).
The results show that the yield of trioxane by H2SO4is not increasing all the time.After2h,the concentration of trioxane is decreasing,and finally stabilizes at 43.01 wt.%.
3.3.VLE experiments
The vapor–liquid equilibrium of trioxane has been made from a 50wt.%aqueous formaldehyde solution in the presence of 2 wt.% IL catalysts or sulfuric acid for the purpose of comparisons.The results are shown in Fig.7.It can be seen from Fig.7a that the yield of trioxane in the vapor phase increase in the order[NOP][BSA]<[NOP][MSA]<[NOP][TfO].Although the yield of trioxane further increased when sulfuric acid was used in place of an IL catalyst,the acidity of the reaction solution also Significantly increased(see Fig.7b).For example,the acidity of the reaction solution and the concentration of formic acid at tr=5 h are 4265 and 1989 g·g?1by[NOP][TfO],4109 and 1722 g·g?1by[NOP][MSA],3915 and 1551 g·g?1by[NOP][BSA],and 25550 and 8714 g·g?1by H2SO4.The order for the equilibrium yield of trioxane using the[NOP]+-based IL catalysts is associated with much more influential factors.In addition to the Hammett acidity function H0as discussed above,the IL can also increase the relative volatility of trioxane and water.Accordingly,using[NOP][TfO]as a catalyst for trioxane synthesis gives rise to the good yield of trioxane.Furthermore,the formation rate of formic acid is approximately the same in reaction solutions using the three ILs.

Fig.7.The yields of trioxane(a)and the acid strength of the reaction solution of the VLE experiments(b)as a function of time using different Br?nsted-acidic IL catalysts:□[NOP][BSA],○[NOP][MSA],△[NOP][TfO],and▽H2SO4(using a 50 wt%aqueous solution of formaldehyde and 2 wt%IL;lines are a guide to eyes).
4.Conclusions
Several water-stable, Br?nsted-acidic ILs that bear sulfonic acid groups in anions have been synthesized. These ILs showed good catalytic activities in preparing trioxane. The conversion rate and the equilibrium yield of trioxane in the reaction solution are inversely related to the Hammett acidity function H0of the aqueous solution of these ILs.And a competitive yield of trioxane and a high selectivity are the main advantages of using these pyrrolidinonium-based ILs in synthesis of trioxane.Furthermore,the high yield and high selectivity of[NOP][MSA],[NOP][TfO]and[NCyP][TfO]make them become competitive candidates in catalyzing the synthesis of trioxane.
[1]J.Chen,H.Y.Song,C.G.Xia,Z.H.Tang,X.Z.Zhang,Z.Li,E.X.Guo,Process for synthesizing trioxymethylene using ionic liquid.U.S.Patent:US,7598402 B2,2009–10-06.
[2]C.G.Xia,Z.H.Tang,J.Chen,X.Z.Zhang,Z.Li,E.X.Guo,Method of synthesizing trioxymethylene from formaldehyde by the catalytic action of an ionic liquid.U.S.Patent:US,7244854 B2,2007–07-17.
[3]A.Curioni,M.Sprik,W.Andreoni,H.Schiffer,J.Hutter,M.Parrinello,Density functional theory-based molecular dynamics simulation of acid-catalyzed chemical reactions in liquid trioxane,J.Am.Chem.Soc.119(1997)7218–7229.
[4]A.Curioni,W.Andreoni,J.Hutter,H.Schiffer,M.Parrinello,Density-functionaltheory-based molecular dynamics study of 1,3,5-trioxane and 1,3-dioxolane protolysis,J.Am.Chem.Soc.116(1994)11251–11255.
[5]D.C.Bassett,Solid state polymerization at{00.1}sub-grain boundaries of trioxane,Nature 215(1967)731–732.
[6]J.Mosamoto,K.Hamanaka,K.Yoshida,H.Nagahara,K.Kagawa,T.Iwaisako,H.Komaki,Synthesis of trioxane using Heteropolyacids as catalyst,Angew.Chem.Int.Ed.39(2000)2102–2104.
[7]H.Benabdallah,D.Olender,Finite element simulation of the wear of polyoxymethylene in pin-on-disc configuration,Wear 261(2006)1213–1224.
[8]R.Luo,X.Zhao,R.Gronner,N.Papke,Low emission polyoxymethylene.U.S.Patent:US,20130324675 A1,2013–12-05.
[9]M.Haubs,D.Feord,J.K.Kurz,J.Lingnau,Process for producing a cyclic acetal in a heterogeneous reaction system.International Patent:WO,2013076290 A1,2013–05-30.
[10]T.Grützner,H.Hasse,N.Lang,M.Siegert,E.Str?fer,Development of a new industrial process for trioxane production,Chem.Eng.Sci.62(2007)5613–5620.
[11]M.Hirohiso,K-s.Okayama,Processes for producing trioxane.European Patent:EP,0789024 B1,2002–02-01.
[12]Y.F.Hu,Z.C.Liu,C.M.Xu,X.M.Zhang,The molecular characteristics dominating the solubility of gases in ionic liquids,Chem.Soc.Rev.40(2011)3802–3823.
[13]A.K.Chakraborti,S.R.Roy,On catalysis by ionic liquids,J.Am.Chem.Soc.131(2009)6902–6903.
[14]T.Welton,Room-temperature ionic liquids.Solvents for synthesis and catalysis,Chem.Rev.99(1999)2071–2083.
[15]Z.C.Liu,X.H.Meng,R.Zhang,C.M.Xu,H.Dong,Y.F.Hu,Reaction performance of Isobutane alkylation catalyzed by a composite ionic liquid at a short contact time,AIChE J.60(2014)2244–2253.
[16]B.A. Rosen, A. Salehi-Khojin, M.R. Thorson, W. Zhu, D.T. Whipple, P.J.A. Kenis, R.I.Masel, Ionic liquid-mediated selective conversion of2to CO at low overpotentials,Science 334(2011)643–644.
[17]Y.F.Hu,H.Z.Huang,Z.Y.Yang,H.R.Zhang,Chinese Patent:201410663695.1,(2014).
[18]C.G.Xia,H.Y.Song,J.Chen,X.Z.Zhang,Z.Li,E.X.Guo,Chinese Patent:CN,101311154 A,(2007).
[19]J.Chen,H.Y.Song,C.G.Xia,Z.H.Tang,Chinese Patent:CN,102020629 A,(2011).
[20]J.Chen,H.Y.Song,C.G.Xia,Z.H.Tang,Chinese Patent:CN,102020630 A,(2011).
[21]J.Chen,H.B.Wang,R.Cheng,Comparison between ionic liquid method and sulfuric acid method of trioxane preparation,Tech.Dev.Chem.Ind.42(2013)60–62.
[22]The catalyst can also increase the relative volatility of trioxane and water and of trioxane and,as the specific interactions between trioxane and the coexisting ions of the catalyst are considerably smaller than those between water(or HO(CH2O)nH)and these ions due to the lack of the–OH group(s)in the molecular structure of trioxane.
[23]J.F.Walker,Formaldehyde,third ed.Reinhold,New York,1970 239.
[24]Z.Y.Yang,Y.F.Hu,Z.X.Wang,Y.Sun,C.C.Jiang,Y.F.Chen,Densities and viscosities of the binary and ternary aqueous solutions of pyrrolidone-based ionic liquids at different temperatures and atmospheric pressure,J.Chem.Eng.Data 59(2014)1094–1104.
[25]M.Kosmulski,J.Gustafsson,J.B.Rosenholm,Thermal stability of low temperature ionic liquids revisited,The rmochim.Acta 412(2004)47–53.
[26]W.H.Awad,J.W.Gilman,M.Nyden,R.H.Harris Jr.,T.E.Sutto,J.Callahan,P.C.Trulove,H.C.DeLong,D.M.Fox,Thermal degradation studies of alkyl-imidazolium salts and their application in nanocomposites,The rmochim.Acta 409(2004)3–11.
[27]M.A.Paul,F.A.Long,H0and related indicator acidity function,Chem.Rev.67(1957)1–45.
[28]C.Thomazeau,H.Olivier-Bourbigou,L.Magna,S.Luts,B.Gilbert,Determination of an acidic scale in room temperature ionic liquids,J.Am.Chem.Soc.125(2003)5264–5265.
[29]Q.Wu,M.Wang,Y.Hao,H.Li,Y.Zhao,Q.Jiao,Synthesis of polyoxymethylene dimethyl ethers catalyzed by Br?nsted acid ionic liquids with Alkanesulfonic acid groups,Ind.Eng.Chem.Res.53(2014)16254–16260.
[30]L.Y.Yin,Y.F.Hu,X.M.Zhang,J.G.Qi,W.T.Ma,The salt effect on the yields of trioxane in reaction solution and in distillate,RSC Adv.5(2015)37,697–37,702.
[31]Y.X.Yu,J.G.Liu,G.H.Gao,Isobaric vapor–liquid equilibria of three aromatic hydrocarbon-tetraethylene glycol binary systems,Fluid Phase Equilib.157(1999)299–307.
[32]J.D.Li,C.X.Chen,J.Wang,Vapor–liquid equilibrium data and their correlation for binary systems consisting of ethanol,2-propanol,1,2-ethanediol and methyl benzoate,Fluid Phase Equilib.169(2000)75–84.
[33]G.Z.Li,Y.F.Hu,Y.S.Liu,G.L.Li,Y.X.Tang,L.H.Wu,C.F.Shi,K.Xu,Isobaricvapor–liquid equilibrium for the ternary system(formaldehyde+1,3-dioxolane+water)at 101.3 kPa,J.Chem.Eng.Data 58(2013)2854–2860.
 Chinese Journal of Chemical Engineering2016年10期
Chinese Journal of Chemical Engineering2016年10期
- Chinese Journal of Chemical Engineering的其它文章
- CFD modeling of a headbox with injecting dilution water in a central step diffusion tube☆
- Interactions between two in-line drops rising in pure glycerin☆
- Hydrodynamics of three-phase fluidization of homogeneous ternary mixture in a conical conduit—Experimental and statistical analysis
- Adsorption of Hg(II)from aqueous solution using thiourea functionalized chelating fiber☆
- Nickel(II)removal from water using silica-based hybrid adsorbents:Fabrication and adsorption kinetics☆
- Reactive dividing wall column for hydrolysis of methyl acetate:Design and control☆
